Rev Cuid. 2024; 15(3): e3612
Abstract
Introduction: Different non-pharmacological interventions have been studied to manage symptoms derived from chemotherapy, but their effectiveness is unknown. Objective: To describe non-pharmacological interventions for managing symptoms secondary to antineoplastic chemotherapy in adults. Materials and Methods: Systematic review of analytical experimental and observational studies (2021 to 2023). The studies were selected, and data was extracted in parallel. Discrepancies were resolved with a third reviewer. The risk of bias was assessed using the Risk of Bias (RoB) tool and The Newcastle-Ottawa Scale (NOS). The literature was synthesized descriptively based on prioritized outcomes. Results: The prioritized outcomes were neutropenia, pain, neuropathy, nausea, vomiting, alopecia, anorexia, and sleep disorders. Out of 7520 references found, 62 were included for analysis. Acupressure showed a possible effect in controlling symptoms such as nausea and vomiting. The intervention with cold on the scalp showed differences in the stages of alopecia severity. Other interventions showed heterogeneity. Discussion: Non-pharmacological interventions have been widely described in observational and experimental studies in the control of side effects of chemotherapy; however, there is homogeneity and a high risk of bias. Conclusion: Acupressure, muscle massage, music therapy, foot baths, and other interventions have been studied for nausea, vomiting, sleep disorders, neutropenia, alopecia, anorexia, pain, and neuropathy as secondary symptoms prioritized by patients. It is necessary to standardize both the interventions and how measure the outcomes.
Keywords: Complementary Therapies; Drug-Related Side Effects and Adverse Reactions; Integrative Oncology; Signs and Symptoms.
Resumen
Introducción: Diferentes intervenciones no farmacológicas se han estudiado para manejar los síntomas derivados de la quimioterapia, pero se desconoce su efectividad. Objetivo: Describir las intervenciones no farmacológicas para el manejo de síntomas secundarios a la quimioterapia antineoplásica en adultos. Materiales y Métodos: Revisión sistemática de estudios experimentales y observacionales analíticos (2021 a 2023). La selección de estudios y extracción de datos se realizó de forma paralela. Las discrepancias se resolvieron con un tercer revisor. Se evaluó el riesgo de sesgo con las herramientas Risk Of Bias (RoB) y The Newcastle-Ottawa Scale (NOS). La síntesis de la literatura se realizó de forma descriptiva por desenlace priorizado. Resultados: Los desenlaces priorizados fueron neutropenia, dolor, neuropatía, náuseas, vomito, alopecia, anorexia y desordenes del sueño. Se encontraron 7520 referencias, 62 incluidas para el análisis. La acupresión mostró un posible efecto en el control de síntomas como las náuseas y vomito. La intervención con frio en el cuero cabelludo mostro diferencias en los estadios de la severidad de alopecia. Las otras intervenciones mostraron heterogeneidad. Discusión: Las intervenciones no farmacológicas han sido ampliamente descritas en estudios observaciones y experimentales en el control de efecto secundarios a la quimioterapia, sin embargo, existe homogeneidad, y alto riesgo de sesgo. Conclusión: Acupresión, masaje muscular, musicoterapia, baño de pies entre otros son las intervenciones que se han estudiado para náuseas, vomito, desordenes del sueño, neutropenia, alopecia, anorexia, dolor y neuropatía como síntomas secundarios priorizados por pacientes. Se requiere estandarizar tanto las intervenciones como la forma de medición de los desenlaces.
Palabras Clave: Terapias Complementarias; Efectos Colaterales y Reacciones Adversas Relacionados con Medicamentos; Oncología integrativa; Signos y Síntomas.
Resumo
Introdução: Diferentes intervenções não farmacológicas têm sido estudadas para o manejo dos sintomas decorrentes da quimioterapia, mas sua eficácia é desconhecida. Objetivo: Descrever intervenções não farmacológicas para o manejo dos sintomas secundários à quimioterapia antineoplásica em adultos. Materiais e Métodos: Revisão sistemática de estudos analíticos experimentais e observacionais (2021 a 2023). A seleção dos estudos e a extração dos dados foram realizadas paralelamente. As discrepâncias foram resolvidas com um terceiro revisor. O risco de viés foi avaliado por meio das ferramentas Risk Of Bias (RoB) e Newcastle-Ottawa Scale (NOS). A síntese da literatura foi realizada de forma descritiva por desfecho priorizado. Resultados: Os desfechos priorizados foram neutropenia, dor, neuropatia, náuseas, vômitos, alopecia, anorexia e distúrbios do sono. Foram encontradas 7.520 referências, 62 incluídas para análise. A acupressão mostrou possível efeito no controle de sintomas como náuseas e vômitos. A intervenção fria no couro cabeludo mostrou diferenças nos estágios de gravidade da alopecia. As demais intervenções apresentaram heterogeneidade. Discussão: Intervenções não farmacológicas têm sido amplamente descritas em estudos observacionais e experimentais no controle dos efeitos colaterais da quimioterapia, porém há homogeneidade e alto risco de viés. Conclusão: Acupressão, massagem muscular, musicoterapia, escalda-pés, entre outras, são as intervenções que têm sido estudadas para náuseas, vômitos, distúrbios do sono, neutropenia, alopecia, anorexia, dor e neuropatia como sintomas secundários priorizados pelos pacientes. É necessário padronizar tanto as intervenções quanto a forma de medir os resultados.
Palavras-Chave: Terapias Complementares; Efeitos Colaterais e Reações Adversas Relacionados a Medicamentos; Oncologia Integrativa; Sinais e Sintomas.
Introduction
In 2020, Globocan reported 19,292,789 new cancer cases worldwide1. Specific treatment regimens have been studied for each type of disease, with chemotherapy being the main intervention2. The incidence of side effects is reported to be 70-80% due to the involvement of rapidly growing cells3. There is evidence of side effects such as nausea, vomiting, alopecia, mucositis, fatigue, constipation, neutropenia, and mood changes, which affect a person's quality of life4,5. Treatment plans include medications to control these symptoms; however, these medications can trigger other secondary symptoms that further impact the quality of life6.
Integrative oncology, in coordination with evidence-based complementary therapies and conventional cancer care, improves patients' quality of life and clinical outcomes. This orientation empowers patients' participation in their treatment7. It has been reported that approximately 50% of cancer patients use complementary and alternative medicine (CAM), and in patients with advanced disease, the prevalence of CAM use can reach 100%7.
The evidence shows a wide variety of non-pharmacological interventions, which presents a challenge to the caregiver when seeking symptom control. This process involves balancing pharmacological treatment, complementation with non-pharmacological interventions, and individual preferences8. This review aims to synthesize the existing evidence on non-pharmacological interventions to control the side effects of chemotherapy, as prioritized by patients and healthcare professionals.
Materials and Methods
The protocol was published in the International Prospective Register of Systematic Reviews (PROSPERO CRD4202017212) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA, 2009)9 guidelines; the analysis database was stored in Mendeley Data10. We included randomized clinical trials (RCTs) and longitudinal analytic observational studies conducted in adults with cancer undergoing treatment that described non-pharmacological interventions to control chemotherapy-related side effects. Studies were only included in the review if the nonpharmacological interventions were delivered by trained personnel. Descriptive studies, cost-effectiveness studies, conference proceedings, systematic reviews, meta-analyses, clinical practice guidelines, letters to the editor, or studies with unanalyzable data or without reported measures of effect, animal studies, or studies in pregnant women were excluded.
Outcome selection and prioritization
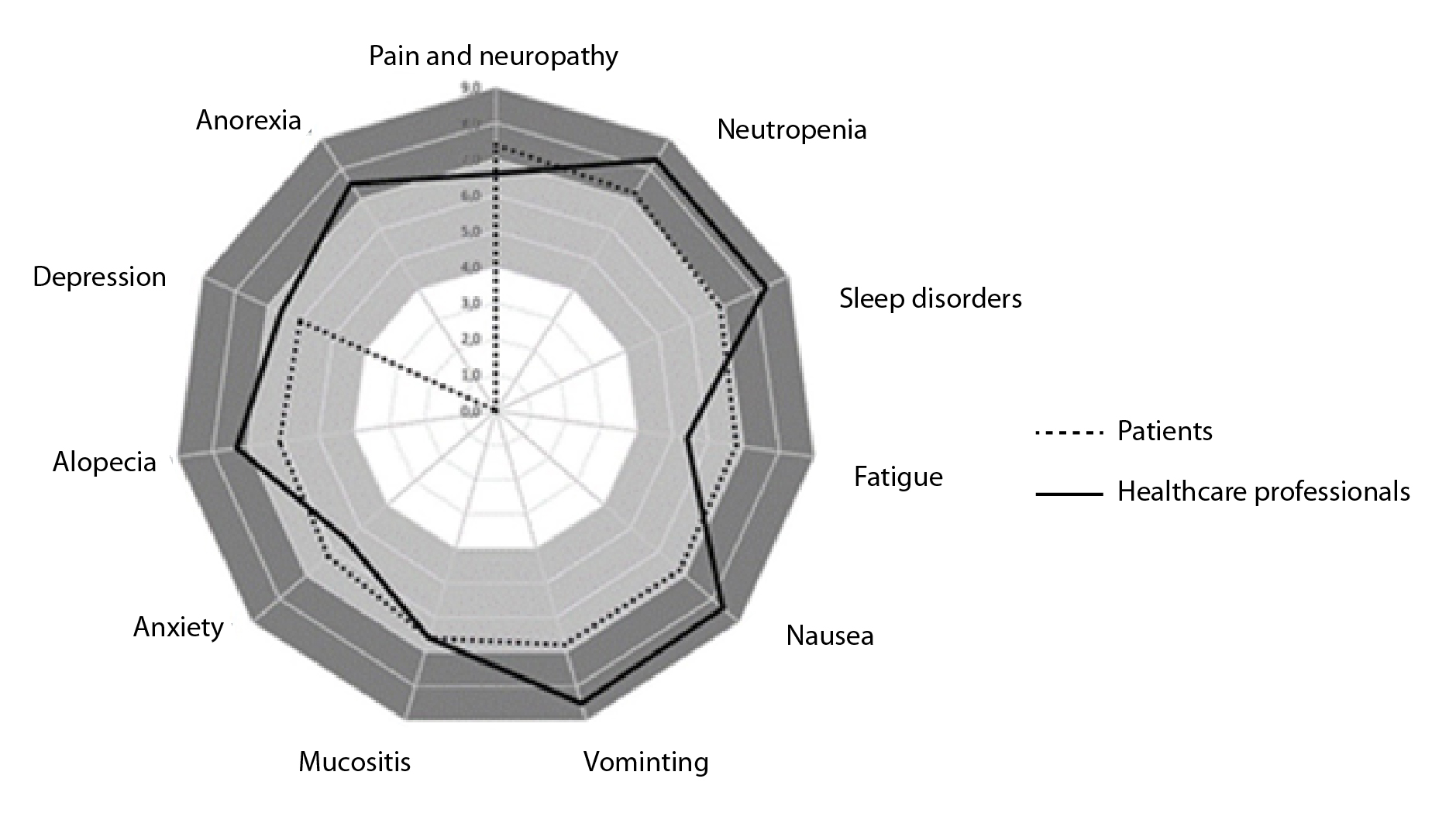
The outcomes were prioritized according to the preferences of patients and health professionals at the time of making a decision about an intervention, including the list described in the literature3,11. Ten cancer experts and chemotherapy patients from a university hospital oncology department were independently asked to prioritize each side effect on a scale of 1 to 9, with 7 to 9 being critical, 4 to 6 being important, and 1 to 3 being of limited importance (according to the GRADE approach12). For this review, outcomes with scores greater than 8 were included (Figure 1).
Search strategy
The electronic databases PubMed/MEDLINE, Ovid Embase, LILACS/Bireme, The Cochrane Library, and Epistemonikos were searched from March 2021 to May 2023. University repositories and reference lists of included studies were also searched. Authors and clinical experts in cancer were also contacted to inquire about possible published studies in this area. The search algorithm was developed using free search terms and the Medical Subject Headings (MeSH) (Table 1).
Table 1. Search strategies used in PubMed, Embase, and LILACS
X
Table 1. Search strategies used in PubMed, Embase, and LILACS
| Database |
Search strategy |
| PubMed |
((((carcinoma chemotherapy OR chemotherapy OR "Chemotherapy, Cancer, Regional Perfusion"[Mesh] OR "Chemotherapy, Adjuvant"[Mesh]) OR "Chemotherapy, Cancer, Regional Perfusion"[Mesh] OR "Chemotherapy, Adjuvant"[Mesh], carcinoma chemotherapy, carcinoma chemotherapy, antineoplastic drugs, "Antineoplastic Agents"[Mesh] OR "Antineoplastic Agents" [Pharmacological Action]) NOT (Child[Mesh] OR oncology pediatric OR pediatric OR child* OR children)) AND (("Oncology Nursing"[Mesh], OR nursing practices, cancer nursing, palliative care nurse, nursing care, nursing interventions, nursing intervention, "Oncology Service, Hospital"[Mesh] OR "Oncology Nursing"[Mesh], "Hospice and Palliative Care Nursing"[Mesh] OR "Oncology Nursing"[Mesh] OR "Nursing Care"[Mesh] OR "Patient Care Planning"[Mesh] OR home care) NOT ((non-pharmacological intervention) OR (non-pharmacological treatment) OR (non-pharmaco*)))) AND ((((((alopecia) OR (((Sleep Wake Disorders) AND (Sleep Disorders, Intrinsic)) AND (((neutropenia) AND ((nausea) AND (vomiting))) AND ((anorexia))))) OR (((neutropenia) AND ((nausea) AND (vomiting))) AND ((anorexia)))) OR ("Metabolic Side Effects of Drugs and Substances"[Mesh] OR "Drug-Related Side Effects and Adverse Reactions"[Mesh] OR secondary side effects)) OR (((Peripheral Nervous System Diseases) AND (Peripheral Neuropathies)) AND (pain))) OR ("Metabolic Side Effects of Drugs and Substances"[Mesh] OR "Drug-Related Side Effects "[Mesh] OR secondary side effects)) |
| Embase |
#11 AND (2020:py OR 2021:py OR 2022:py OR 2023:py) AND 'vomiting'/dm AND ('clinical article'/de OR 'clinical study'/de OR 'clinical trial'/de OR 'clinical trial topic'/de OR 'cohort analysis'/de OR 'controlled clinical trial'/de OR 'controlled study'/de OR 'cross sectional study'/de OR 'double blind procedure'/de OR 'evidence based medicine'/de OR 'evidence based practice'/de OR 'human'/de OR 'human experiment'/de OR 'intervention study'/de OR 'interview'/de OR 'longitudinal study'/de OR 'major clinical study'/de OR 'multicenter study'/de OR 'normal human'/de OR 'observational study'/de OR 'open study'/de OR 'pilot study'/de OR 'prospective study'/de OR 'randomized controlled trial'/de OR 'randomized controlled trial topic'/de) AND ([adult]/lim OR [aged]/lim OR [middle aged]/lim OR [very elderly]/lim OR [young adult]/lim) AND ('article'/it OR 'article in press'/it) |
| LILACS |
(tw:("palliative care nursing" OR "nursing interventions" OR "nursing care" OR "cancer nursing" OR "Enfermagem Oncológica" OR "Hospice and Palliative Care Nursing")) AND (tw:("Efeitos Colaterais e Reações Adversas Relacionados a Medicamentos" OR "efeitos adversos" OR "side effect" OR "adverse effect" OR "adverse events" OR "drug side effect" AND "Tratamento Farmacológico" OR "Tratamento Farmacológico" OR "Antineoplásicos" OR "Tratamento Farmacológico" OR "Antineoplásicos" OR "Quimioterapia Adjuvante")) |
Study selection and data extraction
Two groups of reviewers (Group 1 - MEG-N and ABP; Group 2 - LI/EP and OC) independently screened references found by title and abstract according to the RAYYAN eligibility criteria for systematic reviews13. Two reviewers read full texts for final inclusion. Disagreements were resolved with the assistance of a third reviewer (AB-L). A matrix was created in Microsoft Excel® in which two independent reviewers entered data including authors, year of publication, study’s country of origin, sex, cancer diagnosis, comorbidities, sample size, study population, non-pharmacological intervention used, and measure of the effect in both the experimental and control groups. The authors were contacted to request information on missing data.
Risk of bias assessment
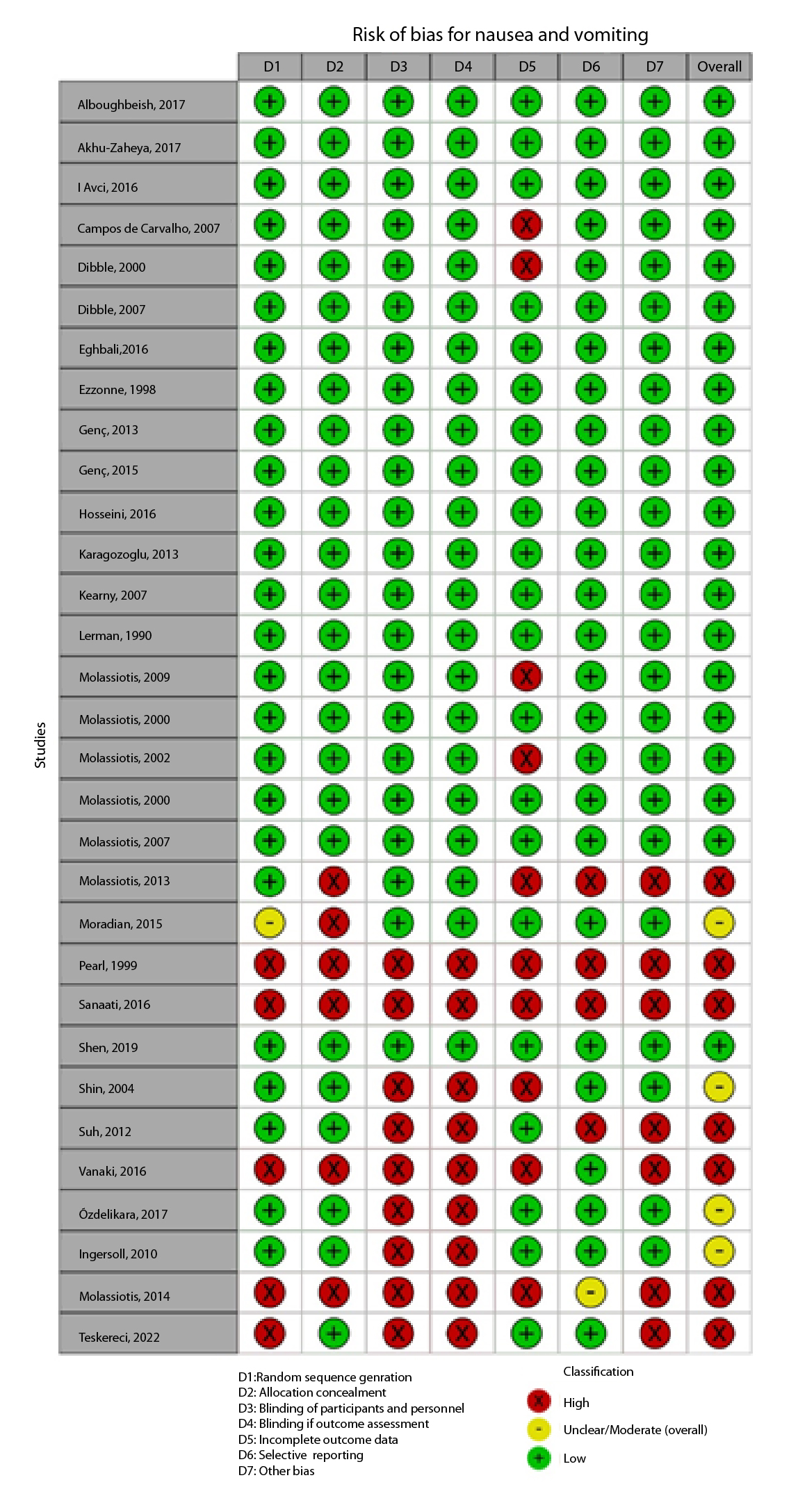
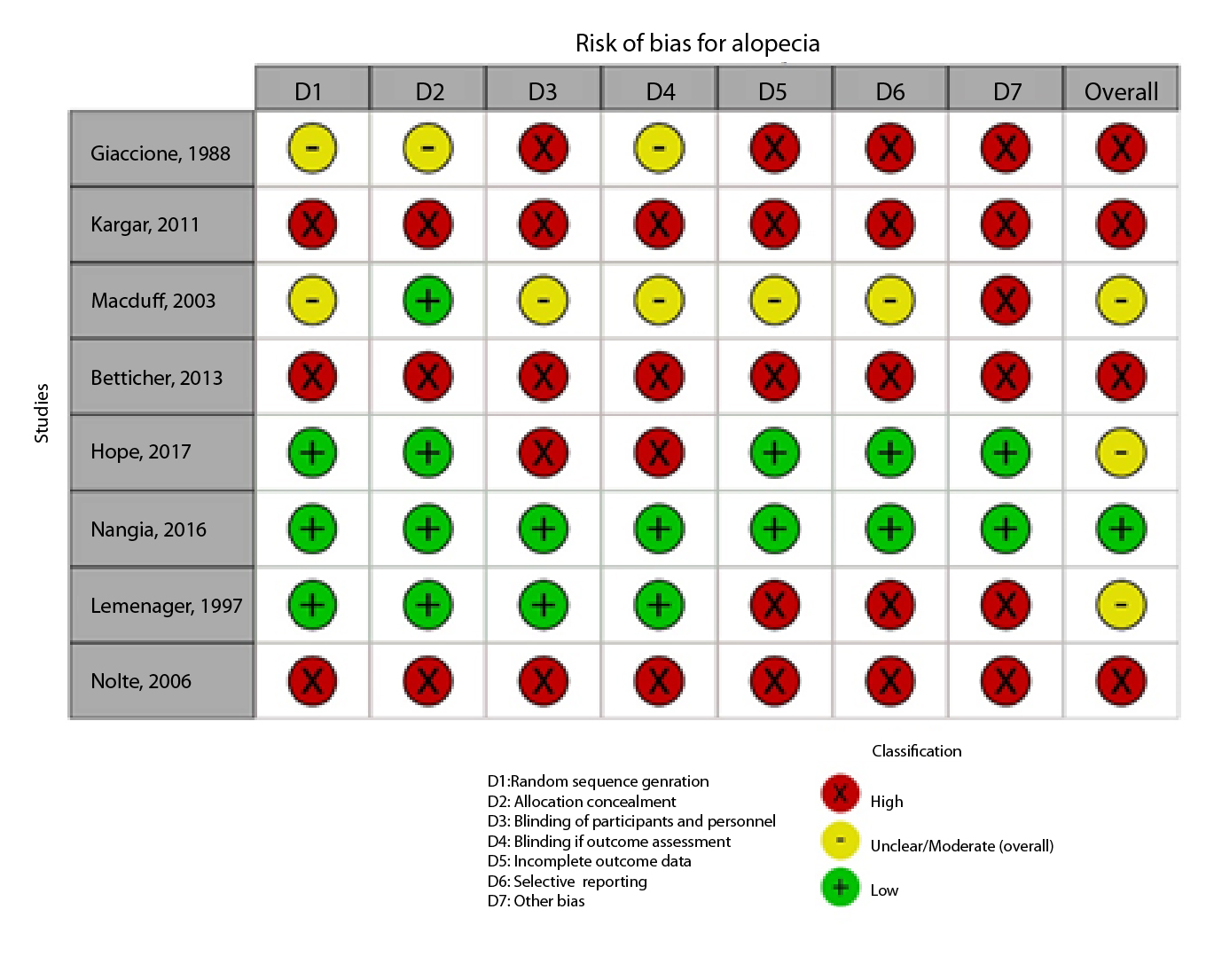
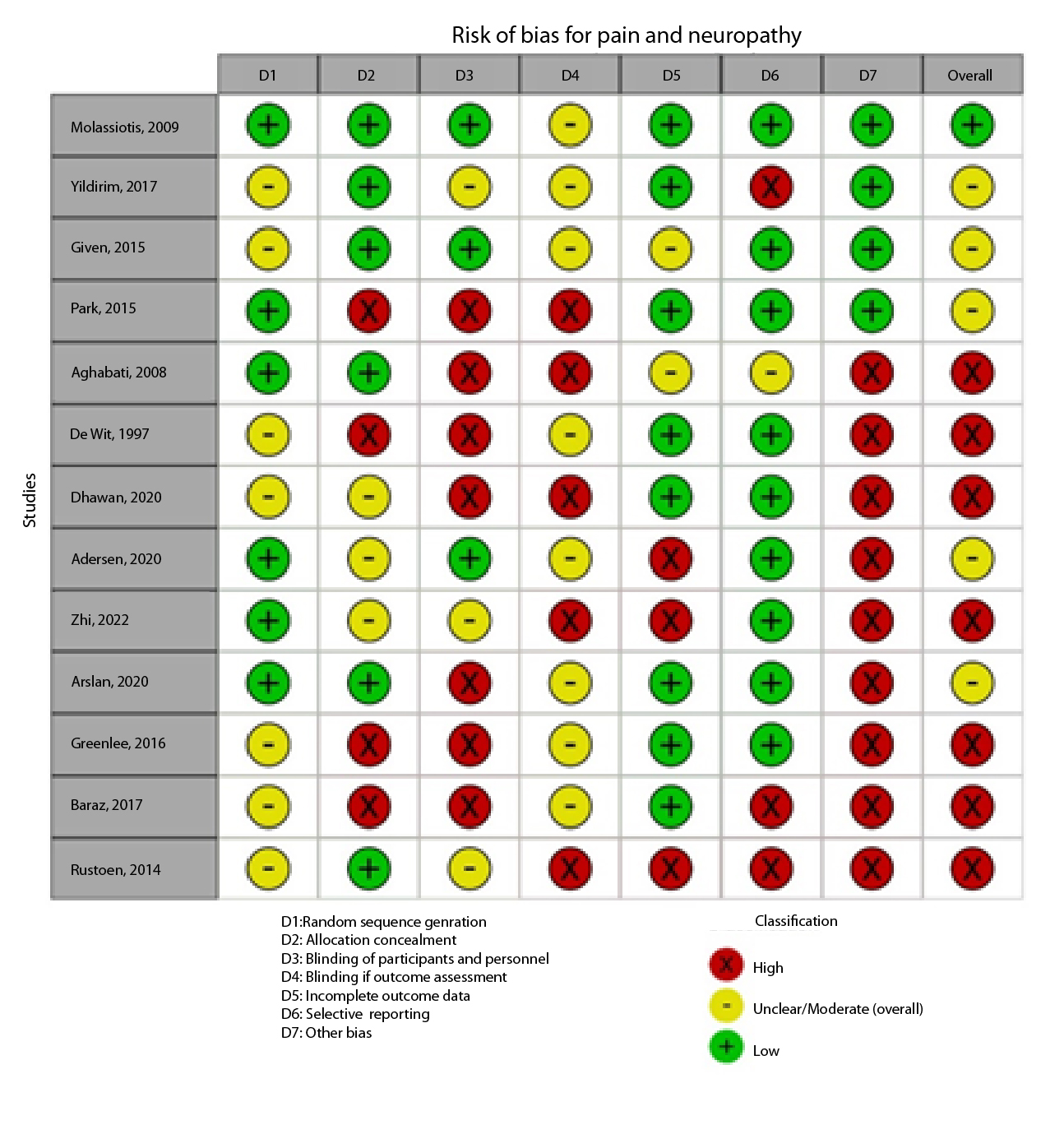
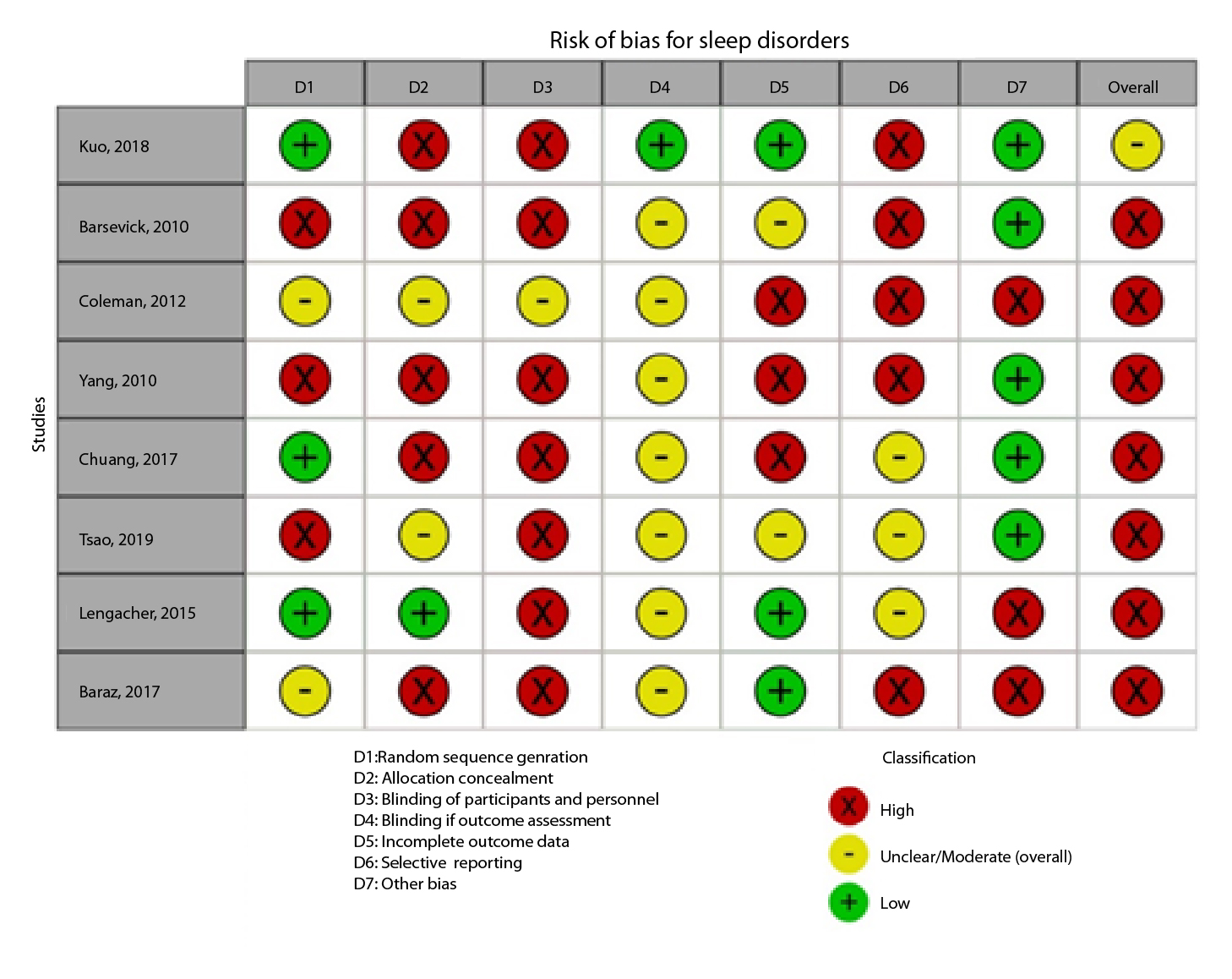
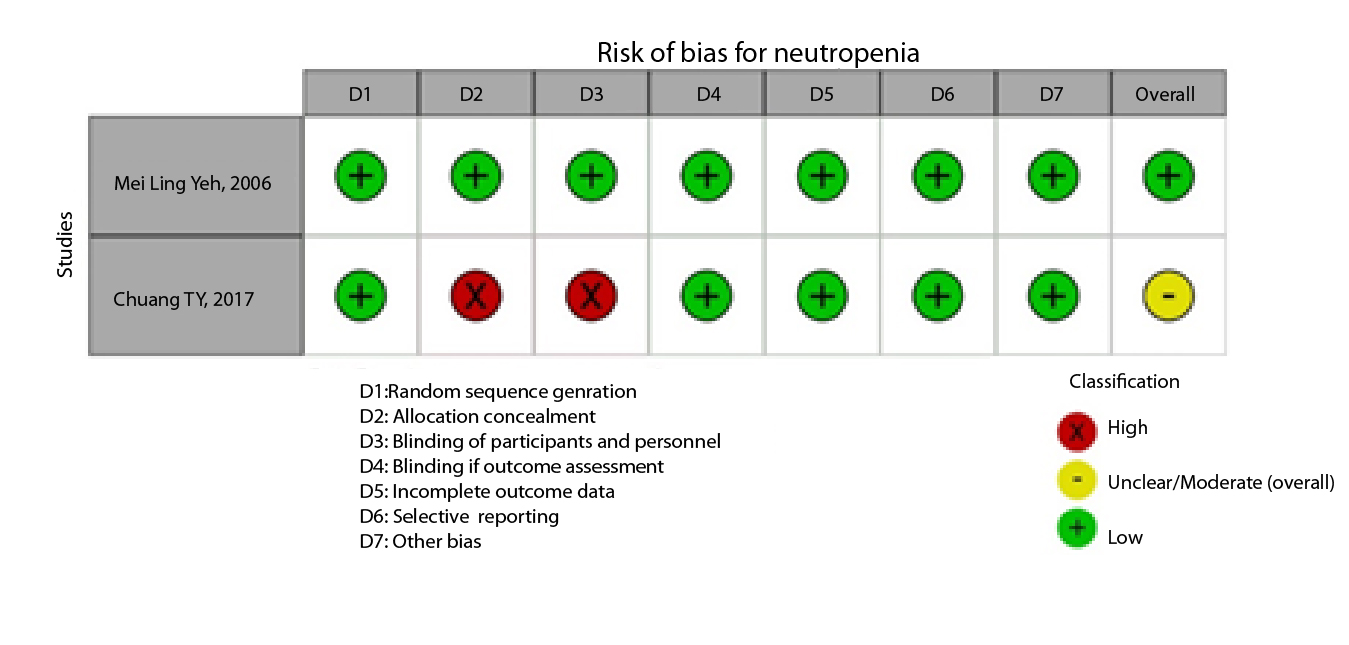
Figure 2A,B,C,D,E, and F show the graphical visualization of the risk of bias for experimental studies assessed with the RoB-2 tool14. Figure 2G shows the risk of bias assessment for analytic observational cohort studies assessed with the Newcastle-Ottawa Scale (NOS)15 (Figure 2).
Synthesis of evidence
Study characteristics were described narratively by outcome. The heterogeneity of the studies was assessed by clinical observation of the population, outcomes and their measurement, and description of the intervention performed10.
Results
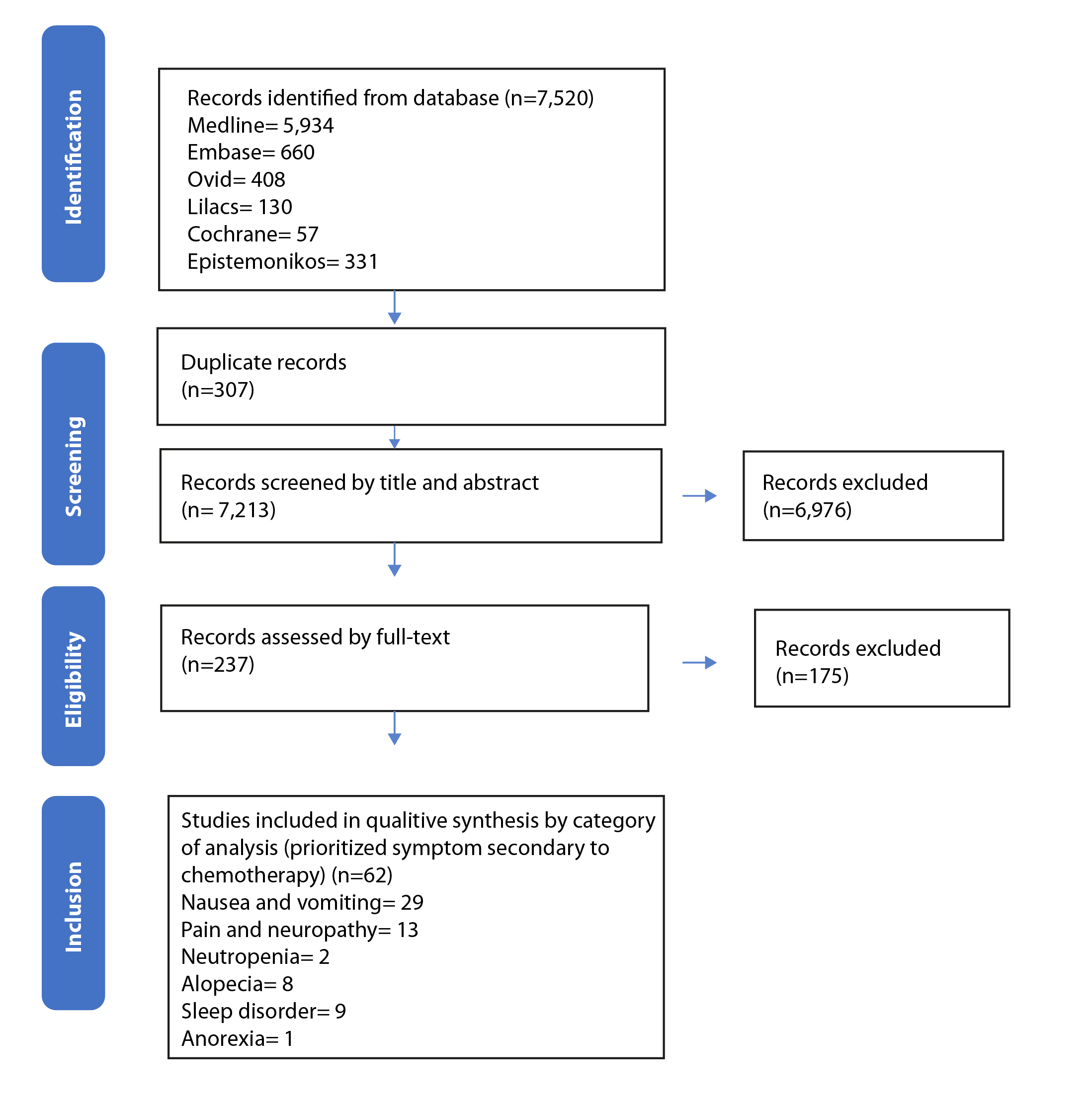
A total of 7,520 references were found, of which 237 were selected for full-text reading. Sixty-two references were included between 1988 and 2023 (Figure 3). Nineteen interventions evaluating 6,613 participants were identified across all studies in the United States, and 4,577 women participated.
Nausea and vomiting
Twenty-nine references were included; 25(89.21%) are RCTs and 4(13.73%) are quasi-experiments with participants between 16 and 96 years of age. We reviewed 4(12.91%) care and counseling programs, 5(16.14%) muscle relaxation techniques, 4(12.95%) guided relaxation with music therapy and imagery, 2(6.44%) natural drinks, 2(6.43%) therapeutic touch and reflexology, 12(38.70%) acupressure at P6 point, and 2(6.41%) hologram bracelets. Studies on interventions such as acupressure were consistent in affirming that there was improvement before and after the intervention; however, they showed high heterogeneity regarding the types of interventions and scales used to measure nausea and vomiting (Table 2).
Anorexia
One RCT conducted in Turkey16 involving women aged 29 to 69 years with stage II or III gynecological cancer was included. The intervention involved a nursing program based on Jean Watson's theory. Nursing professionals visited and followed up with the participants via telephone for 60 to 120 minutes once a week. Information on symptom management was provided and compared with standard hospital management. The authors assessed changes in appetite using the Chemotherapy Symptom Assessment Scale (C-SAS). They found that the intervention group had a lower mean change in appetite of 1.00 SD (0.61) than the control group of 2.00 SD (1.08). This study had a high risk of bias due to the lack of randomization and blinding.
Table 2. Nonpharmacological interventions: Nausea and vomiting outcome
X
Table 2. Nonpharmacological interventions: Nausea and vomiting outcome
| Author, year |
Design |
Population and population size N |
Instrument |
Intervention |
Outcome |
Outcome |
| Before |
After |
| Intervention |
Control |
Intervention |
Control |
| Nursing intervention programs |
| Teskereci, 202216 |
Randomized clinical trial |
Gynecologic cancer N=52 |
Herth Hope Scale |
Nursing program based on Watson's Theory of Human Caring |
Nausea severity Mean (SD) |
|
|
1.0 (0.84) |
3.0 (0.75) |
| Molassioti, 200917 |
Randomized clinical trial |
Colorectal and breast cancer N=164 |
Chemotherapy Symptom Assessment Scale (C-SAS) |
Home nursing care program for symptom management |
Nausea severity Mean (SD) |
|
|
1.0 (0.84) |
3.0 (0.75) |
| Alboughobeish, 201718 |
Quasi-experimental |
Different types of cancer |
|
Mobile care program designed by nurses |
Vomiting frequency. Mean (SD) |
1.8 (1.77) |
1.64 (1.84) |
0.84 (1.37) |
2.48(2.16) |
| Kearney, 200719 |
Randomized clinical trial |
Lung, colorectal, and breast cancer N= 112 |
Advanced symptom management system (ASyMS©) |
Mobile care program designed by nurses |
Severity of vomiting distress. Mean (SD) Severity of nausea distress (SD) |
|
|
0.51 (0,93) 1.23(1.19) |
0.50 (0.81) 1.43 (1.08) |
| Muscle relaxation therapies |
| Campos de Carvalho, 200720 |
Pretest-Posttest |
Different types of cancer N=30 |
Huskisson's visual analog scale |
Muscle relaxation therapy |
Level of nausea Median (IQR)
Level of vomiting. Median (IQR)
|
6.00 (3.75–7.00) 4.00 (2.00-5.25) |
|
4.50 (3.00-6.00)
2.00 (1.00-3.00)
|
|
| Molassioti, 200021 |
Randomized clinical trial |
Breast cancer. N= 8 |
Morrow assessment of nausea and vomiting (MANE) |
Muscle relaxation program |
Nausea duration. Hours
Vomiting duration. Hours
|
7 hours
2.75 hours
|
|
1.5 hours
1.67 hours
|
|
| Lerman, 199022 |
Randomized clinical trial |
Different types of cancer
N=96 |
Emesis Rating Scale |
Muscle relaxation techniques |
Nausea prevalence N (%) |
5(46%) |
3(27%) |
6(54%) |
8(73%) |
| Sensory distraction techniques |
| Ezzonne, 199823 |
Randomized clinical trial |
Bone marrow transplant N= 39 |
Thermometer-shaped visual analog scale |
Music therapy |
Vomiting episodes. Mean (range) |
0.69 (0-4) |
1.73 (0-6) |
0.94 (0-2) |
0.31 (0-2) |
| Hosseini, 201624 |
Quasi-experimental |
Breast cancer N=55 |
Morrow Assessment of Nausea and Vomiting |
Image illustration and audio CD |
a. Nausea severity. Mean (SD)
b. Nausea frequency. Mean (SD)
c. Vomiting severity. Mean (SD)
d. Nausea frequency. Mean (SD)
|
a. 1.91 (1.97)
b. 1.67 (0.88)
c. 0.48 (0.09)
d. 1.10 (0.24)
|
|
a. 2.07 (1.63)
b. 1.91 (0.63)
c. 0.62 (0.05)
d. 0.42 (0.05)
|
|
| Karagozoglu, 201325 |
Randomized clinical trial |
Lung, gastric, and breast cancer
N= 40 |
Visual Analog Scale (VAS) |
Music therapy and visual imagery |
a. Nausea severity. Hours
b. Vomiting severity. Hours
c. Nausea duration. Hours (1-4h)
d. Vomiting duration. Hours (1- 4h)
|
a. 5 (12.5%)
b. 1 (2.5%)
c. 5 (12.5%)
d. 6 (15%)
|
a. 4 (10%)
b. 2 (5%)
c. 8 (20%)
d. 7(17.5%)
|
a. 8 (20%)
b. 9 (22.5%)
c. 7 (17.5%)
d. 8 (20%)
|
a. 2 (5%)
b. 0
c. 8 (20%)
d. 9(22.5%)
|
| Moradian, 201526 |
Randomized clinical trial |
Breast cancer N=99 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Music therapy |
a. Nausea prevalence. Mean (SD)
b. Vomiting prevalence. Mean (SD)
|
|
|
a. 4.31 (4.31)
b. 1.38 (2.70)
|
a.3.0 (3.33)
b.1.46 (3.29)
|
| Substances for oral administration |
| Ingersoll, 201027 |
Randomized clinical trial |
Different types of cancer except for head and neck cancer N=77 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Flavonoid-rich adjunctive treatment (Concord grape juice) |
Nausea and vomiting frequency Mean (SD) |
1.6 (CI 95%: 0.6-2.6) |
1.7 (CI 95%: 0.6-2.8) |
1.6 (CI 95%: 0.3-2.9) |
2.0 (CI 95%: 0.6-3.5) |
| Sanaati, 201628 |
Randomized clinical trial |
Breast cancer N= 65 |
Chemotherapy-induced nausea and
vomiting (CINV)
|
a. Ginger capsules
b. Chamomile capsules
|
a. Number of nausea. Mean difference (SD)
b. Number of vomiting. Mean difference (SD)
|
|
|
a. Nausea: Ginger 1.5845 (0.57)
a. Nausea: Chamomile 0.0769 (0.58)
b. Vomiting: Ginger 0.108 (0.24)
b. Vomiting: Chamomile 0.8394 (0.28)
|
|
| Manual therapies and reflexology |
| Vanaki, 201629 |
Randomized clinical trial |
Breast cancer N= 108 |
Visual Analog Scale (VAS) |
Therapeutic touch: Patterns of energy disturbance in the participant's body |
a. Nausea duration. Mean (SD)
b. Nausea frequency. Median (IQR)
|
|
|
a. 5.36 (2.17)
b. 50.29
|
a. 10.81 (1.77)
b. 31.44
|
| Özdelikara, 201730 |
Randomized clinical trial |
Breast cancer N= 60 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Reflexology |
a. Nausea and vomiting experience Mean (SD)
b. Nausea and vomiting development. Mean (SD)
c. Nausea and vomiting distress Mean (SD)
|
a. Nausea: 2.53 (2.80)
a. Vomiting: 0.83 (1.57)
b. Nausea: 1.83 (2.05)
b. Vomiting: 0.56 (1.07)
c. Nausea: 0.70 (0.83)
c. Vomiting: 0.26 (0.52)
|
a. Nausea: 5.46(4.15)
a. Vomiting: 3.83(4.29)
b. Nausea: 3.70 (2.79)
b. Vomiting: 2.40(2.82)
c. Nausea: 1.76(1.38)
c. Vomiting: 1.43(1.56)
|
a. Nausea: 2.06 (3.33)
a. Vomiting: 0.96 (2.39)
b. Nausea: 1.43 (2.35)
b. Vomiting: 0.63(1.56)
c. Nausea: 0.63(0.99)
c. Vomiting: 0.33(0.84)
|
a. Nausea: 6.56(4.09)
a. Vomiting: 4.0(3.29)
b. Nausea: 4.40(2.82)
b. Vomiting: 2.40(2.02)
c. Nausea: 2.16(1.34)
c. Vomiting: 1.60(1.35)
|
| Acupressure |
| Avcı,201631 |
Randomized clinical trial |
Myeloblastic Leukemia N= 90 |
Visual Analog Scale (VAS) |
Acupressure, P6 point |
a. Nausea severity
b. Vomiting severity
c. Number of nausea episodes
d. Number of vomiting
|
a. 3.3(0.8)
b. 2.4(1.3)
c. 5.5(0.8)
d. 1.0(1.5)
|
a. 6.4 (0.6)
b. 4.6 (0.9)
c. 5.3 (1.3)
d. 1.9 (0.6)
|
a. 2.8(0.6)
b. 1.4(1.3)
c. 5.4 (0,8)
d. 0.6 (0,5)
|
a. 6.5(0.6)
b. 4.6 (0.8)
c. 6.6 (1.9)
d. 2.2
|
| Dibble, 200032 |
Randomized clinical trial |
Breast cancer N=17 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Acupressure, P6 point |
Nausea experience |
|
|
2.83 (1.6) |
3.00 (0.58) |
| Dibble, 200733 |
Randomized clinical trial |
Breast cancer N= 147 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Acupressure, P6 point |
Differences in the incidence of nausea between the experimental and control groups after the intervention. |
|
|
RIN: c2 = 1.19, p = 0.55; NRS: c2 = 1.23, p = 0.55 |
|
| Eghbali, 201634 |
Randomized clinical trial |
Breast cancer N=48 |
Morrow Assessment of Nausea and Emesis (MANE) |
Auricular Acupressure |
a. Nausea intensity. Mean (SD)
b. Nausea frequency. Mean (SD)
c.Vomiting intensity. Mean (SD)
d. Vomiting frequency. Mean (SD)
|
a. 5.63 (3.98)
b. 5.79 (6.4)
c. 1.04 (1.71)
d. 0.79 (1.33)
|
a. 3.71 (4.05)
b. 3.54 (5.31)
c. 2.29 (4.71)
d. 2.08 (5.29)
|
a. 2.08 (3.3)
b. 1.85 (3.1)
c. 0.79 (2.15)
d. 0.54 (1.49)
|
a. 7.54 (4.14)
b. 6.85 (7.25)
c. 3.71 (3.24)
d. 2.06 (2.06)
|
| Genç, 201335 |
Quasi-experimental |
Lung, breast and cervical cancer N=64 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Acupressure, P6 point |
Nausea and vomiting experience. Z (P value) |
|
|
Z=-3.88 P:0.0001
Experimental vs. Placebo: P<0.05
|
Z=-3.15
P: 0.0001
|
| Genç, 201536 |
Quasi-experimental |
Breast cancer N=64 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Acupressure, P6 point |
a. Nausea experience
b. Vomiting experience
c. Nausea occurrence
d. Vomiting occurrence
|
a. 4.71 (3.53)
b. 3.96 (3.18)
c. 3.28 (2.45)
d. 2.56 (2.28)
|
a.5.57 (3.47)
b.4.78 (2.85)
c.3.84 (2.42)
d.3.15(1.90)s
|
a. 1.87 (2.60)
b. 0.46 (1.64)
c. 1.25 (1.77)
d. 0.34 (1.12)
|
a. 4.75 (2.59)
b. 0.31 (0.89)
c. 3.12(1.73)
d. 0.21 (0.60)
|
| Molassiotis, 200737 |
Randomized clinical trial |
Breast cancer N=50 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Acupressure, P6 point |
a. Nausea experience
b. Vomiting experience
c. Nausea occurrence
d. Vomiting occurrence
e. Nausea distress
f. Vomiting distress
|
a. 0.87 (2.2)
b. 0.66 (2.6)
c. 0.66 (1.6)
d. 0.53 (2.1)
e. 0.20 (0.6)
f. 0.12 (0.5)
|
a. 1.46 (3.1)
b. 0.94 (2.7)
c. 2.16 (2.4)
d. 0.66 (1.9)
e. 0.55 (1.0)
f. 0.28 (0.8)
|
a. 2.72 (3.1)
b. 0.2 (0.5)
c. 1.20 (2.6)
d. 0.13 (0.5)
e. 0.27 (0.6)
f. 0.31 (0.4)
|
a. 2.5 (3.4)
b. 0.5 (1.5)
c. 1.94 (2.3)
d. 0.22 (0.6)
e. 0.55 (1.1)
f. 0.67 (0.9)
|
| Molassiotis, 201338 |
Randomized clinical trial |
Different types of cancer N=500 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Acupressure, P6 point |
a. Nausea and vomiting experience. Median (IQR)
b. Nausea frequency N (%)
c. Vomiting frequency. N (%)
|
a.1.0 (0.0-7.50)
b.79 (63%)
c.109 (87%)
|
a.1.43 (0.0-8.57)
b.69 (59%)
c.100 (85%)
|
a. 0.00 (0.0-9.86)
b. 70 (78%)
c. 71 (88%)
|
a. 1.14 (0.0-9.14)
b. 50 (62%)
|
| Molassiotis, 201439 |
Randomized clinical trial |
Different types of cancer N=334 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Acupressure, P6 point |
a. Nausea experience (range 0 to 12). Median (IQR) |
1.0 (2.97 – 7.50) |
1.43 (3.71 – 8.57) |
0.00 (1.82 – 9.86) |
1.14 (4.00– 9.14) |
| Shen, 201940 |
Quasi-experimental |
Lung cancer N=70 |
Morrow Assessment of Nausea and Emesis (MANE) |
Acupressure, P6 point |
a. Nausea severity. Mean (SD)
b. Vomiting severity. Mean (SD)
|
a. 2.94 (0.8)
b. 0.4 (0.1)
|
a. 2.94 (0.9)
b. 1.06 (1.4)
|
a. 0.46 (0.7)
b. 0.03 (0.2)
|
a.2.66 (0.8)
b.0.8 (1.3)
|
| Shin, 200441 |
Randomized clinical trial |
Gastric cancer N=40 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Acupressure, P6 point |
a. Severity. Mean (SD)
b. Duration. Mean (SD)
c. Frequency. Mean (SD)
|
a. 1.55 (3.42)
b. 0.45 (1.36)
c. 0.10 (0.45)
|
a. 3.85 (6.38)
b. 0.65 (1.46)
c. 0.10 (0.45)
|
a. 6.05 (2.85)
b. 1.70 (2.49)
c. 0.30 (0.73)
|
a.9.55 (5.47)
b. 4.25 (3.27)
c. 0.90 (1.33)
|
| Suh, 201242 |
Randomized clinical trial |
Breast cancer N=120 |
Rhodes Index of Nausea, Vomiting and Retching (INVR) |
Acupressure, P6 point |
a. Level of nausea and vomiting. Media (DE) |
7.97 (5.1) |
12.09(9.44) |
3.12 (4.3) |
9.17 (7.58) |
| Akhu-Zaheya, 201743 |
Randomized clinical trial |
Different types of cancer N=224 |
Functional Living Index-Emesis (FLIE), Chemotherapy-induced nausea and vomiting (CINV) |
Hologram bracelets |
a. Vomiting frequency. Mean (SD)
b. Nausea severity. Mean (SD)
c. Vomiting severity. Mean (SD)
|
a. 0.26 (1.27)
b. 1.00 (2.14)
c. 0.44 (1.65)
|
a. 0.46 (1.46)
b.1.09 (2.17)
c. 0.72 (1.97)
|
a. 0.31(1.33)
b. 1.82 (2.99)
c. 0.59 (1.93)
|
a.0.59 (1.45)
b. 2.91 (2.97)
c. 1.28 (2.75)
|
| Pearl, 199944 |
Randomized clinical trial |
Gynecologic cancer N=32 |
Not reported |
Transcutaneous stimulation bracelet |
Report of reduced vomiting intensity |
|
|
71% |
21% |
Alopecia
Eight studies evaluated non-pharmacological interventions to control alopecia, such as scalp cooling with hypothermic caps, and one study used videos on makeup and wigs. Five studies used WHO criteria to evaluate the effect of scalp cooling on reducing alopecia. The other studies used instruments such as the Dean scale, the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, and the breast cancer stem cells (BC SCs) to assess the efficacy of the intervention on hair loss. In general, these studies have a high risk of bias, and scalp cooling shows a possible effect on reducing alopecia compared to placebo (Table 3 ).
Table 3. Non-pharmacological interventions: alopecia outcome
X
Table 3. Non-pharmacological interventions: alopecia outcome
| Author, year |
Design |
Population and population size N |
Instrument |
Intervention |
Outcome |
Outcome |
| Before |
After |
| Intervention |
Control |
Intervention |
Control |
| Betticher, 201345 |
Non-randomized controlled study |
Different types of cancer N= 167 |
WHO alopecia grading (I: slight and regular hair loss, II: moderate hair loss, III: complete but reversible hair loss, IV: complete and irreversible hair loss) |
Scalp cooling Paxman® PSC-2 machine (PAX) |
Reduction of alopecia grades III and IV % |
|
|
80% |
78% |
| Giaccone, 198846 |
Randomized clinical trial |
Different types of cancer
N= 39 |
Unclear. A 4-point grading scale is used: 0 no hair loss, 1 minimal hair loss (<25%), 2 moderate hair loss (25-50%), and 3 severe alopecia (>50%). |
Hypothermia Cap (commercially available as Spenco Hypothermia Cap-Spenco Medical Corporation, Texas) |
Hair loss (reduction of alopecia grade 3) |
|
|
Grade 0:5
Grade 1:2
Grade 2:1
Grade 3:11
|
Grade 0:0
Grade 1:0
Grade 2:1
Grade 3:15
|
| Kargar, 201147 |
Non-randomized experiment |
Unspecified cancers. N=63 |
WHO alopecia scale |
Scalp cooling system |
Hair loss (reduction of alopecia grades 3-4) |
Grade 1-2: 24 (77.4%)
Grade 3-4: 7 (22.6%)
|
Grade 1-2: 12 (38.7%)
Grade 3-4: 19 (61.3%)
|
Grade 1-2: 15 (50%)
Grade 3-4: 15 (50%)
|
Grade 1-2: 8 (25%)
Grade 3-4: 24 (75%)
|
| Macduff, 200348 |
Randomized clinical trial |
Breast cancer N=30 |
WHO alopecia scale |
Cool cap |
Hair loss (increase from grade 0 to 2) |
Grades 0 a 2: 73% |
Grades 0 a 2: 23% |
Grades 0 a 2: 25% |
Grades 0 a 2: 0% |
| Nangía, 201649 |
Randomized clinical trial |
Breast cancer N=182 |
CTCAE v. 4.0 grade 0 (No hair loss), grade 1 (Hair loss of <50% of normal but it does not require wearing a wig). Failure was defined as CTCAE v4.0 grade 2 (Hair loss of >50% normal and it requires wearing a wig). |
Scalp cooling |
Efficacy: success in hair preservation N (%) |
|
|
N=95
Grade 0: 48 (50.5%)
Grade 1: 5 (5.3%)
Grade >2: 47 (49.5%)
|
N=47
Grade 0: 0 (0%)
Grade 1: 0 (0%)
Grade >2: 47 (100%)
|
| Lemenage, 199750 |
Randomized clinical trial |
Different types of cancer N=98 |
WHO alopecia grading
Grade 0: No hair loss
Grade 1: Slight hair loss
Grade 2: moderate hair loss
Grade 3: complete but reversible hair loss
Grade 4: complete and irreversible hair loss
|
Cool cap |
Efficacy: Degree of alopecia less than 2 N (%) |
|
|
Grades 0-1: 83 (85.60%) |
Grades 2-4: 14 (14.4%) |
| Nolte, 200651 |
Randomized clinical trial |
Gynecologic cancer N=187 |
Breast cancer stem cells (BC SCs) (Secord & Jourand, 1953). |
45-minute video featuring makeup techniques and suggestions for women's hairstyles and headpieces. |
Body image perception |
|
|
2.24 (0.61) |
2.17 (0.53) |
| Rugo, 201752 |
Randomized clinical trial |
Breast cancer N=182 |
Dean scale |
Scalp colling |
Efficacy: success in hair preservation N (%) |
|
|
67 (66.3%) |
0 (0%) |
Pain and neuropathy
A total of 1,403 patients, aged 15 to 86 years, were observed in 14 studies. Interventions included educational programs, acupuncture, physical activity, psychological therapies, natural substance applications, massages, and foot baths. Pain and neuropathy were measured using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTC), Numerical Pain Scale (NPS), the Dutch Language Version of the McGill Pain Questionnaire (MPQ-DLV), and Symptom Experience Scale. Of the total, 6 (42.81%) studies evaluated disease-related pain, and 8 (57.20%) studies evaluated platinum or taxane chemotherapy-related neuropathy (Table 4). These studies have a high risk of bias due to selective reporting of outcomes, lack of concealment, and lack of blinding. Interventions such as home-based care nursing programs and acupuncture were demonstrated to reduce mean pain and neuropathy when comparing pre- and post-intervention measurements.
Table 4. Non-pharmacological interventions: pain and neuropathy outcome
X
Table 4. Non-pharmacological interventions: pain and neuropathy outcome
| Author, year |
Design |
Population and population size N |
Instrument |
Intervention |
Outcome |
Outcome |
| Before |
After |
| Intervention |
Control |
Intervention |
Control |
| Nursing intervention programs |
| Molassiotis, 200917 |
Randomized clinical trial |
Colorectal and breast cancer N=164 |
CTCAE Toxicity Rating Scale (NIH/NCI) |
Home care nursing program |
Toxicity grading Mean |
NR |
NR |
2.9 |
6.3 |
| Rustoen, 201453 |
Randomized clinical trial |
Different types of cancer with bone metastasis N=179 |
Care Needs Assessment (CNA) |
Nursing program for pain management (PRO-SELF) |
Pain Mean |
3.6 |
3.7 |
2.7 |
3.1 |
| De Wit, 199754 |
Randomized clinical trial |
Different types of cancer N=313 |
McGill Pain Questionnaire (MPQ-DLV) |
Pain education program |
Pain % |
58.4 |
55.9 |
39.4 |
16.9 |
| Muscle exercises |
| Aghabati, 200855 |
Randomized clinical trial |
Cancer patients N=90 |
Care Needs Assessment (CNA) |
Therapeutic touch |
Pain Mean |
1.9 |
0.02 |
1 |
0 |
| Miladinia, 201756 |
Randomized clinical trial |
Acute Leukemia N=64 |
Care Needs Assessment (CNA) |
Slow-Stroke Back Massage (SSBM) |
Pain Mean |
6.5 |
6 |
4.8 |
6.3 |
| Dhawan, 202057 |
Randomized clinical trial |
Different types of cancer N=45 |
Chemotherapy-induced peripheral neuropathy (CIPN) |
Muscle strengthening exercises |
Neuropathy Mean |
132.5 |
129.3 |
83.1 |
140.8 |
| Self-affirmation |
| Yildirim, 201758 |
Randomized clinical trial |
Different types of cancer N=140 |
Edmonton Symptom Assessment System (ESAS) |
Self-affirmation |
Pain Mean |
0.66 |
1.31 |
0.09 |
2.03 |
| Given, 201559 |
Randomized clinical trial |
Different types of cancer N=113 |
Symptom experience scale |
Supportive care |
Pain n (%)/mean |
29(69)/7.3 |
30(63)/6.8 |
19(54)/3.3 |
25(58)/4.4 |
| Foot bath |
| Park, 201560 |
Quasi-experimental |
Colorectal and gastric cancer N=48 |
CTCAE Toxicity Rating Scale (NIH/NCI) |
Foot bath |
Neurotoxicity grades 2 and 3 n (%) |
24(100) |
24(100) |
20(83) |
21(87.5) |
| Neural gliding |
| Andersen, 202061 |
Randomized clinical trial |
Breast cancer N=61 |
Disability of the Arm, Shoulder and Hand (DASH) questionnaire |
Nerve gliding exercises |
Neuropathy. Mean |
44.1 |
44.8 |
40.6 |
45.9 |
| Acupuncture |
| Zhi, 202262 |
Randomized clinical trial |
Different types of cancer N=63 |
Quantitative Sensory Testing (QST) |
Acupuncture |
Thermal neuropathy n/mean |
21/46.31 |
19/46.31 |
17/47.12 |
16/46.96 |
| Arslan, 202063 |
Randomized clinical trial |
Colorectal and gastric cancer. N=60 |
CTCAE Toxicity Rating Scale (NIH/NCI) |
Henna application |
Neuropathy Mean |
65 |
67.9 |
40.9 |
68.4 |
| Greenlee, 201664 |
Randomized clinical trial |
Breast cancer N=63 |
Net Promoter Score de 4 (NPS-4 score) |
Acupuncture |
Neuropathy Mean |
16.8 |
35.2 |
7.9 |
18 |
Sleep disorders
Nine studies evaluated non-pharmacological interventions to control sleep disorders. Acupressure, telephone follow-up programs, home exercises, relaxation therapies such as foot baths, mindfulness therapies, back massages, and Chinese practices like Chan-Chuang qigong have been studied for their effectiveness in improving sleep quality. However, it is observed that interventions such as acupressure and physical exercise improve sleep quality when comparing intervention groups with post-intervention control groups (Table 5).
Table 5. Non-pharmacological Interventions: Sleep Disorders
X
Table 5. Non-pharmacological Interventions: Sleep Disorders
| Author, year |
Design |
Population and population size N |
Instrument |
Intervention |
Outcome |
Outcome |
| Before |
After |
| Intervention |
Control |
Intervention |
Control |
| Acupressure |
| Tsao, 201965 |
Quasi-experimental |
Ovarian cancer N=60 |
PSQI- Pittsburgh Sleep Quality Index |
Acupressure |
Sleep quality Mean |
2.5 |
2.24 |
2.4 |
4.05 |
| Kuo, 201866 |
Randomized clinical trial |
Ovarian cancer N=40 |
PSQI-Pittsburgh Sleep Quality Index |
Acupressure |
Sleep quality Mean |
13.2 |
12.25 |
4.21 |
12.75 |
| Telephone follow-up programs |
| Barsevick, 201011 |
Randomized clinical trial |
Different types of cancer N=276 |
PSQI-Pittsburgh Sleep Quality Index |
Telephone follow-ups and education |
Sleep quality Mean |
8.01 |
7.83 |
7.96 |
8.24 |
| Physical exercise programs |
| Coleman, 201267 |
Randomized clinical trial |
Multiple myeloma N=187 |
Actigraphy* |
Physical exercise program |
Sleep quality Mean |
79.7 |
81.39 |
77.79 |
76.57 |
| Foot bathing |
| Yang, 201068 |
Randomized clinical trial |
Gynecologic cancers N=50 |
Verran y Snyder-Halpern Sleep Scale |
Warm-water footbath |
Sleep quality Mean |
805.5 |
743 |
944.9 |
763.2 |
| Movement and relaxation practices |
| Chuang, 201769 |
Randomized clinical trial |
Non-Hodgkin lymphoma N=96 |
Verran y Snyder-Halpern Sleep Scale |
Practice of Chan-Chuang qigong |
Sleep quality Mean |
657 |
79.7 |
922.9 |
77.19 |
| Yang, 202170 |
Cohort |
Ovarian cancer N=389 |
PSQI- Pittsburgh Sleep Quality Index |
Exercise and cognitive behavioral therapy |
Sleep quality Mean |
13.94 |
14.76 |
14.29 |
14.37 |
| Reich, 201571 |
Randomized clinical trial |
Breast cancer N=79 |
PSQI- Pittsburgh Sleep Quality Index |
Mindfulness |
Sleep quality Mean |
7.97 |
8.39 |
6.91 |
6.91 |
| Baraz, 201756 |
Randomized clinical trial |
Acute leukemia N=64 |
PSQI- Pittsburgh Sleep Quality Index |
Slow-Stroke Back Massage on Symptom (SSBM) |
Sleep quality Mean |
12.23 |
9.7 |
12.1 |
12.37 |
*Actigraphy: An instrument used to monitor sleep and wakefulness patterns.
Neutropenia
Two studies analyzed 167 participants diagnosed with neutropenia, defined as a decrease in neutrophils following chemotherapy treatment, and administered Chan-Chuang qigong therapy for 21 minutes over 21 days. This technique includes mind and body relaxation, with white blood cell counts measured before and after the procedure. The studies have a high risk of bias due to the non-randomization of participants, but the intervention showed an increase in white blood cell counts after the intervention (Table 6).
Table 6. Non-pharmacological Interventions: Neutropenia
X
Table 6. Non-pharmacological Interventions: Neutropenia
| Author, year |
Design |
Population and population size N |
Instrument |
Intervention |
Outcome |
Outcome |
| Before |
After |
| Intervention |
Control |
Intervention |
Control |
| Mei Ling Yeh, 200672 |
Quasi-experimental |
Breast cancer
N: 67 |
SYSMEX9000 automatic blood analyzer |
Chan-Chuang qi-gong therapy |
WBC count
Hemoglobin
Platelets
|
1.955 μL
11.42 g/dL
189,500 μL
|
1.955 μL
11.32 g/dL
194,523 μL
|
> 416.25 μL
< 0.27 g/dL
> 92,531.25μL
|
> 810.57 μL
< 0.43g/dL
> 67,057.14 μL
|
| Chuang TY, 201769 |
Randomized clinical trial |
Non-Hodgkin lymphoma
N:100
|
Beckman automatic blood analyzer |
Chan-Chuang qi-gong therapy |
WBC count
Hemoglobin
Platelets
|
4,731.46 μL
(SD 2,074.34 μL)
11.64 g/dl
(SD 2.03 g/dL)
173,479.17
(SD 96,707.49 μL)
|
5,482.29 μL
(SD 3,460.63 μL)
11.39 g/dL
(SD 2.03 g/dL)
200,645.83 μL
(94,867.32 μL)
|
6,478.33 μL
(SD 4,222.05 μL)
11.97 g/dL
(SD 2.06 g/dL)
177,395.83 μL
(SD 80,056.29 μL)
|
4,150.42 μL
(SD 2,142.67 μL)
11.07 g/dL
(SD 2.15 g/dL)
179,250.00 μL
(SD 80,795.38 μL)
|
Discussion
This review described non-pharmacological interventions for controlling the primary side effects of chemotherapy with a high degree of heterogeneity and internal validity among the studies. This is consistent with some studies stating that non-pharmacological interventions are complementary to medical treatments; however, they emphasize the lack of valid evidence to present the effect of these interventions as complementary to pharmacological treatments73,74.
The review described several types of non-pharmacological interventions to address the side effects of chemotherapy. These interventions include education and exercise programs, hypothermia devices, acupressure techniques, music therapy, traditional Chinese medicine techniques, relaxation techniques, foot baths, and transcutaneous electrical nerve stimulation75,76.
Nurse-led home-based patient education programs are designed to manage symptoms. These non-pharmacological interventions have shown measurable differences in pain levels before and after the intervention54,59. A need was identified to standardize educational programs and to know the content and indicators for pain assessment26,76,79. However, for patients with multiple symptoms, these processes should be accompanied by psychological support and strengthening of mental health to ensure beneficial application and results in the control of the symptoms.
Holistic medical systems such as acupressure have been studied extensively. This review found that acupressure consistently reduced nausea and vomiting compared to standard care in all measurements36. This result is consistent with the study by Lee A et al.78, who conducted a review and found that acupressure at the P6 point has a moderate effect compared to placebo, although the studies have limitations in terms of variation in effects and methodological quality. However, when comparing acupressure with antiemetics, no difference in the incidence of nausea and vomiting was observed. Therefore, it can be concluded that the available evidence may support a combined therapy of P6 point stimulation and antiemetic drugs rather than drug prophylaxis alone and that further high-quality trials are needed76-79.
Manipulative and body-based practices, such as muscle relaxation therapies, reflexology, and therapeutic touch, along with sensory intervention techniques like music therapy and guided imagery, have been described and evaluated with positive effects80,81; however, the reported studies record wide variability of populations, techniques, and study periods regarding outcomes such as pain, nausea and vomiting76-81. The main limitation of these studies was the lack of control for confounding factors, such as the use of medications and other therapies and individual perception of the symptom.
It is important to consider that these types of studies are valuable in building the body of evidence that will later support evidence-based recommendations82. The literature consistently states that acupressure is a complementary technique and does not replace traditional treatment79. The reported studies agree that environmental factors and the use of patients' unreported therapies limit the evaluation of interventions; hence, there is a need to identify what type of interventions patients are conducting.
The immune system's vulnerability to opportunistic infections and the extended duration of treatment make neutropenia a priority in evaluating non-pharmacological interventions. Chan-Chuang qigong therapy has been evaluated in people diagnosed with cancer69,72 and showed an increase in white blood cell count before and after the intervention. However, variables such as time, comorbidities, and treatments must be controlled to estimate the true effect of this intervention.
Alopecia is one of the secondary symptoms that compromise biological, psychological, emotional, and social aspects, affecting the health status of people who suffer from it and is increasingly becoming a priority outcome for the well-being of patients83,84. Video tutorials for makeup, wig styling, and scalp cooling are techniques that have been increasingly reported in recent years to mitigate these effects and improve the quality of life for patients. There is a need to further clarify alopecia measurement strategies with validated scales for different populations.
This review included observational and experimental studies, giving a broad overview of the interventions reviewed. These results suggest some implications for clinical practice and future research. First, each of these interventions and their results should be considered with caution since the representativeness of the populations and the standardization of the techniques used can only be generalized to patients with characteristics similar to those studied in the included studies. Secondly, for research purposes, it is highly recommended that future reviews focus on interventions by symptom clusters85. The search strategies used in this review enabled us to capture the broadest selection of relevant literature according to the side effects of chemotherapy using distinct search terms. The included studies showed low methodological quality and evidence that interventions could have a real effect on controlling various symptoms, as evidenced by acupressure on symptoms such as nausea and vomiting, sleep disorders, pain, and neuropathy. The findings of this review highlight the gaps in the available literature and emphasize the importance of further documenting the effect of non-pharmacological interventions on chemotherapy side effects.
Conclusion
Prioritizing side effects for patients guides care plans for individuals. Non-pharmacological interventions such as acupressure, Chinese therapies such as Chan-Chuang qigong, muscle relaxation therapies, and nursing intervention programs have been evaluated and described with evidence for nausea and vomiting, pain and neuropathy, sleep disorders, alopecia, neutropenia, and anorexia. However, there is still high variability in the type of intervention, outcomes measuring, and lack of statistical power, making it difficult to estimate the effects of these interventions. Research with methodological rigor and standardization of these interventions is needed to validate their effects on these outcomes.
Conflict of interest: The authors declare no conflicts of interest.
Funding: Own funds.
Acknowledgment: We thank Nurse Ana Beatriz Pizarro for her contribution to the selection of the review articles.
References
X
Referencias
The International Agency for Research on Cancer (IARC). Global Cancer Observatory [Internet]. Iarc.fr. [cited 2023 May 5]. Available from: https://gco.iarc.fr/
X
Referencias
Cefalo M, Ruggiero A, Maurizi P, Attinà G, Arlotta A, Riccardi R. Pharmacological management of chemotherapy-induced nausea and vomiting in children with cancer. J Chemother. 2009;21(6):605–10. https://doi.org/10.1179/joc.2009.21.6.605
X
Referencias
Sinclair S, Beamer K, Hack T, McClement S, Raffin Bouchal S, Chochinov H, et al. Sympathy, empathy, and compassion: A grounded theory study of palliative care patients’ understandings, experiences, and preferences. Palliat Med. 2017;31(5):437–47. https://doi.org/10.1177/0269216316663499
X
Referencias
Mao J, Ismaila N, Bao T, Barton D, Ben-Arye E, Garland E, et al. Integrative Medicine for Pain Management in Oncology: Society for Integrative Oncology-ASCO Guideline. J Clin Oncol. 2022;40(34):3998–4024https://doi.org/10.1200/JCO.22.01357
X
Referencias
Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339. https://doi.org/10.1136/bmj.b2700
X
Referencias
Gómez Neva ME, Buitrago Lopez A, Pulido E, Ibáñez L, Caroprese O. Intervenciones no-farmacológicas para efectos priorizados por pacientes, de quimioterapia antineoplásica: revisión sistemática, Mendeley Data, V1. 2023 https://doi.org/10.17632/v2g4p7h4fd.1
X
Referencias
Zhang Y, Alonso-Coello P, Guyatt G, Yepes-Nuñez J, Aki E, Hazlewood G, et al. GRADE Guidelines: 19. Assessing the certainty of evidence in the importance of outcomes or values and preferencesd- Risk of bias and indirectness. J Clin Epidemiol. 2019; 111:94–104. https://doi.org/10.1016/j.jclinepi.2018.01.013
X
Referencias
Sterne J, Savović J, Page M, Elbers R, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366. https://doi.org/10.1136/bmj.l4898
X
Referencias
Teskereci G, Yangın H, Kulakaç Ö. Effects of a nursing care program based on the theory of human caring on women diagnosed with gynecologic cancer: a pilot study from Turkey. Journal of Psychosocial Oncology. 2022;40(1):45–61. https://doi.org/10.1080/07347332.2021.1878317
X
Referencias
Molassiotis A, Brearley S, Saunders M, Craven O, Wardley A, Farrell C, et al. Effectiveness of a home care nursing program in the symptom management of patients with colorectal and breast cancer receiving oral chemotherapy: A randomized, controlled trial. Journal of Clinical Oncology. 2009;(36):6191-8https://doi.org/10.1200/JCO.2008.20.6755
X
Referencias
Kearney N, McCann L, Norrie J, Taylor L, Gray P, McGee-Lennon M, et al. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS©) in the management of chemotherapy-related toxicity. Supportive Care in Cancer. 2009;17(4):437–44. https://doi.org/10.1007/s00520-008-0515-0
X
Referencias
Molassiotis A. A pilot study of the use of progressive muscle relaxation training in the management of post-chemotherapy nausea and vomiting. Eur J Cancer Care (Engl). 2000;9(4):230–4. https://doi.org/10.1046/j.1365-2354.2000.00220.x
X
Referencias
Lerman C, Rimer B, Blumberg B, Cristinzio S, Engstrom P, MacElwee N, et al. Effects of coping style and relaxation on cancer chemotherapy side effects and emotional responses. Cancer Nurs. 1990;13(5):308 -315. https://pubmed.ncbi.nlm.nih.gov/2245418/
X
Referencias
Hosseini M, Tirgari B, Forouzi MA, Jahani Y. Guided imagery effects on chemotherapy induced nausea and vomiting in Iranian breast cancer patients. Complement Ther Clin Pract. 2016;25:8–12. http://dx.doi.org/10.1016/j.ctcp.2016.07.002
X
Referencias
Karagozoglu S, Tekyasar F, Yilmaz FA. Effects of music therapy and guided visual imagery on chemotherapy-induced anxiety and nausea-vomiting. J Clin Nurs. 2013;22(1–2):39–50. https://doi.org/10.1111/jocn.12030
X
Referencias
Moradian S, Walshe C, Shahidsales S, Ghavam Nasiri MR, Pilling M, Molassiotis A. Nevasic audio program for the prevention of chemotherapy induced nausea and vomiting: A feasibility study using a Randomized clinical trial design. European Journal of Oncology Nursing. 2015;19(3):282–91. https://doi.org/10.1016/j.ejon.2014.10.016
X
Referencias
Ingersoll GL, Wasilewski A, Haller M, Pandya K, Bennett J, He H, et al. Effect of concord grape juice on chemotherapy-induced nausea and vomiting: Results of a pilot study. Oncol Nurs Forum. 2010;37(2):213–21. https://doi.org/10.1188/10.ONF.213-221
X
Referencias
Sanaati F, Najafi S, Kashaninia Z, Sadeghi M. Effect of Ginger and Chamomile on Nausea and Vomiting Caused by Chemotherapy in Iranian Women with Breast Cancer. Asian Pac J Cancer Prev. 2016;17(8):4125-4129. https://pubmed.ncbi.nlm.nih.gov/27644672/
X
Referencias
Vanaki Z, Matourypour P, Gholami R, Zare Z, Mehrzad V, Dehghan M. Therapeutic touch for nausea in breast cancer patients receiving chemotherapy: Composing a treatment. Complement Ther Clin Pract. 2016;22:64–8. http://dx.doi.org/10.1016/j.ctcp.2015.12.004
X
Referencias
Özdelikara A, Tan M. The effect of reflexology on chemotherapy-induced nausea, vomiting, and fatigue in breast cancer patients. Asia Pac J Oncol Nurs. 2017;4(3):241–9. https://doi.org/10.4103/apjon.apjon_15_17
X
Referencias
Dibble S, Luce J, Cooper B, Israel J, Cohen M, Nussey B, et al. Acupressure for chemotherapy-induced nausea and vomiting: a randomized clinical trial. Oncol Nurs Forum. 2007;34(4):1–8. https://doi.org/10.1188/07.ONF.xxx-xxx
X
Referencias
Eghbali M, Yekaninejad M, Varaei S, Jalalinia S, Samimi M, Sa’atchi K. The effect of auricular acupressure on nausea and vomiting caused by chemotherapy among breast cancer patients. Complement Ther Clin Pract. 2016;24:189–94. https://doi.org/10.1016/j.ctcp.2016.06.006
X
Referencias
Genç F, Tan M. The effect of acupressure application on chemotherapy-induced nausea, vomiting, and anxiety in patients with breast cancer. Palliative & Supportive Care. 2015;13(2):275-84. https://doi.org/10.1017/S1478951514000248
X
Referencias
Genç A, Can G, Aydiner A. The efficiency of the acupressure in prevention of the chemotherapy-induced nausea and vomiting. Support Care Cancer. 2013;21(1):253-261. https://doi.org/10.1007/s00520-012-1519-3
X
Referencias
Molassiotis A, Helin AM, Dabbour R, Hummerston S. The effects of P6 acupressure in the prophylaxis of chemotherapy-related nausea and vomiting in breast cancer patients. Complement Ther Med. 2007;15(1):3-12. https://doi.org/10.1016/j.ctim.2006.07.005
X
Referencias
Molassiotis A, Russell W, Hughes J, Breckons M, Lloyd-Williams M, Richardson J, et al. The effectiveness and cost-effectiveness of acupressure for the control and management of chemotherapy-related acute and delayed nausea: Assessment of Nausea in Chemotherapy Research (ANCHoR), a randomised controlled trial. Health Technol Assess. 2013;17(26):1-114. https://doi.org/10.3310/hta17260
X
Referencias
Molassiotis A, Russell W, Hughes J, Breckons M, Lloyd-Williams M, Richardson J, et al. The effectiveness of acupressure for the control and management of chemotherapy-related acute and delayed nausea: A Randomized clinical trial. J Pain Symptom Manage. 2014;47(1):12-25. https://doi.org/10.1016/j.jpainsymman.2013.03.007
X
Referencias
Shen CH, Yang LY. The Effects of Acupressure on Meridian Energy as well as Nausea and Vomiting in Lung Cancer Patients Receiving Chemotherapy. https://doi.org/10.1177/1099800416683801
X
Referencias
Shin YM, Kim TI, Shin MS, Juon HS. Effect of Acupressure on Nausea and Vomiting During Chemotherapy Cycle for Korean Postoperative Stomach Cancer Patients. Cancer Nursing. 2004;27(4):267-274. https://doi.org/10.1097/00002820-200407000-00002
X
Referencias
Suh EE. The effects of P6 acupressure and nurse-provided counseling on chemotherapy-induced nausea and vomiting in patients with breast cancer. Oncol Nurs Forum. 2012;39(1):e1-9. https://doi.org/10.1188/12.ONF.E1-E9
X
Referencias
Akhu-Zaheya LM, Khater WA, Lafi AY. The effectiveness of hologram bracelets in reducing chemotherapy-induced nausea and vomiting among adult patients with cancer. Cancer Nurs. 2017;40(2):E17–29. https://doi.org/10.1097/NCC.0000000000000374
X
Referencias
Pearl ML, Fischer M, McCauley DL, Valea FA, Chalas E. Transcutaneous electrical nerve stimulation as an adjunct for controlling chemotherapy-induced nausea and vomiting in gynecologic oncology patients. Cancer Nurs. 1999;22(4):307–311. https://doi.org/10.1097/00002820-199908000-00008
X
Referencias
Betticher DC, Delmore G, Breitenstein U, Anchisi S, Zimmerli-Schwab B, Müller A, et al. Efficacy and tolerability of two scalp cooling systems for the prevention of alopecia associated with docetaxel treatment. Support Care Cancer. 2013;21:2565–2573. https://doi.org/10.1007/s00520-013-1804-9
X
Referencias
Giaccona G, Di Giulio F, Morandini MP, Calciati A. Scalp hypothermia in the prevention of doxorubicin-induced hair loss. Cancer Nurs. 1988;11(3):170-173. https://pubmed.ncbi.nlm.nih.gov/3401852/
X
Referencias
Kargar M, Sarvestani RS, Khojasteh HN, Heidari MT. Efficacy of penguin cap as scalp cooling system for prevention of alopecia in patients undergoing chemotherapy. J Adv Nurs. 2011;67(11):2473–7. https://doi.org/10.1111/j.1365-2648.2011.05668.x
X
Referencias
Macduff C, Mackenzie T, Hutcheon A, Melville L, Archibald H. The effectiveness of scalp cooling in preventing alopecia for patients receiving epirubicin and docetaxel. Eur J Cancer Care. 2003;12(2):154–61. https://doi.org/10.1046/j.1365-2354.2003.00382.x
X
Referencias
Nangia J, Tao W, Osborne C, Niravath P, Otte K, Papish S, et al. Effect of a Scalp Cooling Device on Alopecia in Women Undergoing Chemotherapy for Breast Cancer: The SCALP Randomized Clinical Trial. JAMA. 2017;317(6):596-605. https://jamanetwork.com/journals/jama/fullarticle/2601500
X
Referencias
Lemenager M, Lecomte S, Bonneterre ME, Bessa E, Dauba J, Bonneterre J. Effectiveness of cold cap in the prevention of docetaxel-induced alopecia. Eur J Cancer. 1997;33(2):297-300. https://doi.org/10.1016/S0959-8049(96)00374-7
X
Referencias
Nolte S, Donnelly J, Kelly S, Conley P, Cobb R. A randomized clinical trial of a videotape intervention for women with chemotherapy-induced alopecia: a gynecologic oncology group study. Oncol Nurs Forum. 2006;33(2):305-11. https://pubmed.ncbi.nlm.nih.gov/16518446/
X
Referencias
Rustøen T, Valeberg BT, Kolstad E, Wist E, Paul S, Miaskowski C. A randomized clinical trial of the efficacy of a self-care intervention to improve cancer pain management. Cancer Nurs. 2014;37(1):34–43. https://doi.org/10.1097/NCC.0b013e3182948418
X
Referencias
De Wit R, Van Dam F, Zandbelt L, Van Buuren A, Van der Heijden K, Leenhouts G, et al. A Pain Education Program for chronic cancer pain patients: Follow-up results from a Randomized clinical trial. Pain. 1997;73(1):55–69. https://doi.org/10.1016/s0304-3959(97)00070-5
X
Referencias
Aghabati N, Mohammadi E, Pour Esmaiel Z. The Effect of Therapeutic Touch on Pain and Fatigue of Cancer Patients Undergoing Chemotherapy. Evid Based Complement Alternat Med. 2010;7(3):375https://doi.org/10.1093/ecam/nen006
X
Referencias
Miladinia M, Baraz S, Shariati A, Malehi AS. Effects of Slow-Stroke Back Massage on Symptom Cluster in Adult Patients with Acute Leukemia: Supportive Care in Cancer Nursing. Cancer Nurs. 2017;40(1):31–8. https://doi.org/10.1097/NCC.0000000000000353
X
Referencias
Dhawan S, Andrews R, Kumar L, Wadhwa S, Shukla G. A Randomized clinical trial to Assess the Effectiveness of Muscle Strengthening and Balancing Exercises on Chemotherapy-Induced Peripheral Neuropathic Pain and Quality of Life Among Cancer Patients. Cancer Nurs. 2020;43(4):269–80. https://doi.org/10.1097/NCC.0000000000000693
X
Referencias
Yildirim M, Gulsoy H, Batmaz M, Ozgat C, Yesilbursali G, Aydin R et al. Symptom management: The effects of self-affirmation on chemotherapy-related symptoms. Clin J Oncol Nurs. 2017;21(1):E15-22. https://doi.org/10.1188/17.CJON.E15-E22
X
Referencias
Given B, Given CW, McCorkle R, Kozachik S, Cimprich B, Rahbar MH, et al. Pain and fatigue management: results of a nursing randomized clinical trial. Oncol Nurs Forum. 2002;29(6):949–56. https://doi.org/10.1188/02.ONF.949-956
X
Referencias
Andersen Hammond E, Pitz M, Steinfeld K, Lambert P, Shay B. An Exploratory Randomized Trial of Physical Therapy for the Treatment of Chemotherapy-Induced Peripheral Neuropathy. Neurorehabil Neural Repair. 2020;34(3):235–46. https://doi.org/10.1177/1545968319899918
X
Referencias
Zhi WI, Baser RE, Talukder D, Mei YZ, Harte SE, Bao T. Mechanistic and thermal characterization of acupuncture for chemotherapy-induced peripheral neuropathy as measured by quantitative sensory testing. Breast Cancer Res Treat. 2023;197(3):535–45. https://doi.org/10.1007/s10549-022-06846-3
X
Referencias
Arslan S, Zorba Bahceli P, İlik Y, Artaç M. The preliminary effects of henna on chemotherapy-induced peripheral neuropathy in women receiving oxaliplatin-based treatment: A parallel-group, randomized, controlled pilot trial. Eur J Oncol Nurs. 2020;48. https://doi.org/10.1016/j.ejon.2020.101827
X
Referencias
Greenlee H, Crew KD, Capodice J, Awad D, Buono D, Shi Z, et al. Randomized sham-controlled pilot trial of weekly electro-acupuncture for the prevention of taxane-induced peripheral neuropathy in women with early-stage breast cancer. Breast Cancer Res Treat. 2016;156(3):453–64.https://doi.org/10.1007/s10549-016-3759-2
X
Referencias
Tsao Y, Creedy DK. Auricular acupressure: reducing side effects of chemotherapy in women with ovarian cancer. Supportive Care in Cancer. 2019;27(11):4155–4163.https://doi.org/10.1007/s00520-019-04682-8
X
Referencias
Kuo HC, Tsao Y, Tu HY, Dai ZH, Creedy DK. Pilot Randomized clinical trial of auricular point acupressure for sleep disturbances in women with ovarian cancer. Res Nurs Health. 2018;41(5):469–79. https://doi.org/10.1002/nur.21885
X
Referencias
Coleman EA, Goodwin JA, Kennedy R, Coon SK, Richards K, Enderlin C, et al. Effects of Exercise on Fatigue, Sleep, and Performance: A Randomized Trial. Oncol Nurs Forum. 2012;39(5):468-77. https://doi.org/10.1188/12.ONF.468-477
X
Referencias
Yang HL, Chen XP, Lee KC, Fang FF, Chao YF. The effects of warm-water footbath on relieving fatigue and insomnia of gynecologic cancer patients on chemotherapy. Cancer Nurs. 2010;33(6):454–60. https://doi.org/10.1097/NCC.0b013e3181d761c1
X
Referencias
Chuang T, Yeh M, Chung Y. A nurse facilitated mind-body interactive exercise (Chan-Chuang qigong) improves the health status of non-Hodgkin lymphoma patients receiving chemotherapy: Randomised controlled trial. Int J Nurs Stud. 2017;69:25–33. https://doi.org/10.1016/j.ijnurstu.2017.01.004
X
Referencias
Yang W, Xi J, Guo L, Cao Z. Nurse-led exercise and cognitive-behavioral care against nurse-led usual care between and after chemotherapy cycles in Han Chinese women of ovarian cancer with moderate to severe levels of cancer-related fatigue: A retrospective analysis of the effectiveness. Medicine. 2021;100(44):e27317. https://doi.org/10.1097/MD.0000000000027317
X
Referencias
Reich RR, Lengacher CA, Klein TW, Newton C, Shivers S, Ramesar S, et al. A Randomized clinical trial of the Effects of Mindfulness-Based Stress Reduction (MBSR[BC]) on Levels of Inflammatory Biomarkers Among Recovering Breast Cancer Survivors. Biol Res Nurs. 2017;19(4):456–64. https://doi.org/10.1177/1099800417707268
X
Referencias
Yeh M, Lee T, Chen H, Chao T. The influences of Chan-Chuang qi-gong therapy on complete blood cell counts in breast cancer patients treated with chemotherapy. Cancer Nurs. 2006;29(2):149–55. https://doi.org/10.1097/00002820-200603000-00012
X
Referencias
Turner L, Lau V, Neeson S, Davies M. International Exchange Programs: Professional Development and Benefits to Oncology Nursing Practice. Clin J Oncol Nurs. 2019;23(4):439–42. https://doi.org/10.1188/19.CJON.439-442
X
Referencias
Morehead A, Salmon G. Efficacy of Acupuncture/Acupressure in the Prevention and Treatment of Nausea and Vomiting Across Multiple Patient Populations: Implications for Practice. Nurs Clin North Am. 2020;55(4):571–80. https://doi.org/10.1016/j.cnur.2020.07.001
X
Referencias
De Paolis G, Naccarato A, Cibelli F, D’Alete A, Mastroianni C, Surdo L, et al. The effectiveness of progressive muscle relaxation and interactive guided imagery as a pain-reducing intervention in advanced cancer patients: A multicentre randomised controlled non-pharmacological trial. Complement Ther Clin Pract. 2019;34:280–7.https://doi.org/10.1016/j.ctcp.2018.12.014
X
Referencias
Mora DC, Overvåg G, Jong MC, Kristoffersen AE, Stavleu DC, Liu J, et al. Complementary and alternative medicine modalities used to treat adverse effects of anti-cancer treatment among children and young adults: a systematic review and meta-analysis of Randomized clinical trials. BMC Complement Med Ther. 2022;22(1):97. https://doi.org/10.1186/s12906-022-03537-w
X
Referencias
Coelho A, Parola V, Cardoso D, Bravo ME, Apóstolo J. Use of non-pharmacological interventions for comforting patients in palliative care: a scoping review. JBI Database System Rev Implement Rep. 2017;15(7):1867–904. https://doi.org/10.11124/JBISRIR-2016-003204
X
Referencias
Freites-Martinez A, Shapiro J, Goldfarb S, Nangia J, Jimenez JJ, Paus R, et al. Hair disorders in patients with cancer. J Am Acad Dermatol. 2019;80(5):1179–96. https://doi.org/10.1016/j.jaad.2018.03.055
X
Referencias
Mao J, Pillai G, Andrade C, Ligibel J, Basu P, Cohen L, et al. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J Clin. 2022;72(2):144–64. https://doi.org/10.3322/caac.21706
-
The International Agency for Research on Cancer (IARC). Global Cancer Observatory [Internet]. Iarc.fr. [cited 2023 May 5]. Available from: https://gco.iarc.fr/
-
Instituto Nacional del Cáncer. Tipos de tratamiento. Consulta: mayo 05, 2022. Disponible en:https://www.cancer.gov/espanol/cancer/tratamiento/tipos
-
American Cancer Society. Efectos secundarios de la quimioterapia. Consulta: mayo 05, 2022. Disponible en: https://www.cancer.org/es/tratamiento/tratamientos-y-efectos-secundarios/tipos-de-tratamiento/quimioterapia/efectos-secundarios-de-la-quimioterapia.html
-
Instituto Nacional del Cáncer. Náuseas y vómitos relacionados con el tratamiento del cáncer (PDQ®)–Versión para profesionales de salud – NCI. Consulta: Mayo 05, 2022. Disponible en: https://www.cancer.gov/espanol/cancer/tratamiento/efectos-secundarios/nauseas/nauseas-pro-pdq
-
Cefalo M, Ruggiero A, Maurizi P, Attinà G, Arlotta A, Riccardi R. Pharmacological management of chemotherapy-induced nausea and vomiting in children with cancer. J Chemother. 2009;21(6):605–10. https://doi.org/10.1179/joc.2009.21.6.605
-
Cope DG. Clinical updates in nausea and vomiting. Semin Oncol Nurs. 2022;38(1):151249. https://doi.org/10.1016/j.soncn.2022.151249
-
Sinclair S, Beamer K, Hack T, McClement S, Raffin Bouchal S, Chochinov H, et al. Sympathy, empathy, and compassion: A grounded theory study of palliative care patients’ understandings, experiences, and preferences. Palliat Med. 2017;31(5):437–47. https://doi.org/10.1177/0269216316663499
-
Mao J, Ismaila N, Bao T, Barton D, Ben-Arye E, Garland E, et al. Integrative Medicine for Pain Management in Oncology: Society for Integrative Oncology-ASCO Guideline. J Clin Oncol. 2022;40(34):3998–4024https://doi.org/10.1200/JCO.22.01357
-
Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339. https://doi.org/10.1136/bmj.b2700
-
Gómez Neva ME, Buitrago Lopez A, Pulido E, Ibáñez L, Caroprese O. Intervenciones no-farmacológicas para efectos priorizados por pacientes, de quimioterapia antineoplásica: revisión sistemática, Mendeley Data, V1. 2023 https://doi.org/10.17632/v2g4p7h4fd.1
-
Barsevick A, Dudley W, Beck S. Cancer-related Fatigue, Depressive Symptoms, and Functional Status: A Mediation Model. Nurs Res. 2006;55(5):366-72. https://doi.org/10.1097/00006199-200609000-00009
-
Zhang Y, Alonso-Coello P, Guyatt G, Yepes-Nuñez J, Aki E, Hazlewood G, et al. GRADE Guidelines: 19. Assessing the certainty of evidence in the importance of outcomes or values and preferencesd- Risk of bias and indirectness. J Clin Epidemiol. 2019; 111:94–104. https://doi.org/10.1016/j.jclinepi.2018.01.013
-
Mourad Ouzzani, Hossam Hammady, Zbys Fedorowicz, Ahmed Elmagarmid. Rayyan — a web and mobile app for systematic reviews. Systematic Reviews. 2016; 5:210. https://doi.org/10.1186/s13643-016-0384-4
-
Sterne J, Savović J, Page M, Elbers R, Blencowe N, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366. https://doi.org/10.1136/bmj.l4898
-
Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. Ottawa Hospital Research Institute. 2011;2(1):1-12 https://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf
-
Teskereci G, Yangın H, Kulakaç Ö. Effects of a nursing care program based on the theory of human caring on women diagnosed with gynecologic cancer: a pilot study from Turkey. Journal of Psychosocial Oncology. 2022;40(1):45–61. https://doi.org/10.1080/07347332.2021.1878317
-
Molassiotis A, Brearley S, Saunders M, Craven O, Wardley A, Farrell C, et al. Effectiveness of a home care nursing program in the symptom management of patients with colorectal and breast cancer receiving oral chemotherapy: A randomized, controlled trial. Journal of Clinical Oncology. 2009;(36):6191-8https://doi.org/10.1200/JCO.2008.20.6755
-
Alboughobeish SZ, Asadizaker M, Rokhafrooz D, Cheraghian B. The effect of mobile-based patient education on nausea and vomiting of patients undergoing chemotherapy. Biomedical Research. 2017;28(19):8172–8 https://www.alliedacademies.org/articles/the-effect-of-mobilebased-patient-education-on-nausea-and-vomiting-of-patients-undergoing-chemotherapy-8580.html
-
Kearney N, McCann L, Norrie J, Taylor L, Gray P, McGee-Lennon M, et al. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS©) in the management of chemotherapy-related toxicity. Supportive Care in Cancer. 2009;17(4):437–44. https://doi.org/10.1007/s00520-008-0515-0
-
De Carvalho EC, Martins FTM, Dos Santos CB. A pilot study of a relaxation technique for management of nausea and vomiting in patients receiving cancer chemotherapy. Cancer Nurs. 2007;30(2):163-167. https://journals.lww.com/cancernursingonline/fulltext/2007/03000/a_pilot_study_of_a_relaxation_technique_for.12.aspx
-
Molassiotis A. A pilot study of the use of progressive muscle relaxation training in the management of post-chemotherapy nausea and vomiting. Eur J Cancer Care (Engl). 2000;9(4):230–4. https://doi.org/10.1046/j.1365-2354.2000.00220.x
-
Lerman C, Rimer B, Blumberg B, Cristinzio S, Engstrom P, MacElwee N, et al. Effects of coping style and relaxation on cancer chemotherapy side effects and emotional responses. Cancer Nurs. 1990;13(5):308 -315. https://pubmed.ncbi.nlm.nih.gov/2245418/
-
Ezzone S, Baker C, Rosselet R, Terepka E. Music as an adjunct to antiemetic therapy. Oncol Nurs Forum. 1998;25(9):1551-1556. https://pubmed.ncbi.nlm.nih.gov/9802051/
-
Hosseini M, Tirgari B, Forouzi MA, Jahani Y. Guided imagery effects on chemotherapy induced nausea and vomiting in Iranian breast cancer patients. Complement Ther Clin Pract. 2016;25:8–12. http://dx.doi.org/10.1016/j.ctcp.2016.07.002
-
Karagozoglu S, Tekyasar F, Yilmaz FA. Effects of music therapy and guided visual imagery on chemotherapy-induced anxiety and nausea-vomiting. J Clin Nurs. 2013;22(1–2):39–50. https://doi.org/10.1111/jocn.12030
-
Moradian S, Walshe C, Shahidsales S, Ghavam Nasiri MR, Pilling M, Molassiotis A. Nevasic audio program for the prevention of chemotherapy induced nausea and vomiting: A feasibility study using a Randomized clinical trial design. European Journal of Oncology Nursing. 2015;19(3):282–91. https://doi.org/10.1016/j.ejon.2014.10.016
-
Ingersoll GL, Wasilewski A, Haller M, Pandya K, Bennett J, He H, et al. Effect of concord grape juice on chemotherapy-induced nausea and vomiting: Results of a pilot study. Oncol Nurs Forum. 2010;37(2):213–21. https://doi.org/10.1188/10.ONF.213-221
-
Sanaati F, Najafi S, Kashaninia Z, Sadeghi M. Effect of Ginger and Chamomile on Nausea and Vomiting Caused by Chemotherapy in Iranian Women with Breast Cancer. Asian Pac J Cancer Prev. 2016;17(8):4125-4129. https://pubmed.ncbi.nlm.nih.gov/27644672/
-
Vanaki Z, Matourypour P, Gholami R, Zare Z, Mehrzad V, Dehghan M. Therapeutic touch for nausea in breast cancer patients receiving chemotherapy: Composing a treatment. Complement Ther Clin Pract. 2016;22:64–8. http://dx.doi.org/10.1016/j.ctcp.2015.12.004
-
Özdelikara A, Tan M. The effect of reflexology on chemotherapy-induced nausea, vomiting, and fatigue in breast cancer patients. Asia Pac J Oncol Nurs. 2017;4(3):241–9. https://doi.org/10.4103/apjon.apjon_15_17
-
Avci HS, Ovayolu N, Ovayolu Ö. Effect of acupressure on nausea-vomiting in patients with acute myeloblastic leukemia. Holist Nurs Pract. 2016;30(5):257–62. https://doi.org/10.1097/HNP.0000000000000161
-
Dibble S, Chapman J, Mack K, Shih A. Acupressure for nausea: results of a pilot study. Oncol Nurs Forum. 2000;27(1);41-7. https://pubmed.ncbi.nlm.nih.gov/10660922/
-
Dibble S, Luce J, Cooper B, Israel J, Cohen M, Nussey B, et al. Acupressure for chemotherapy-induced nausea and vomiting: a randomized clinical trial. Oncol Nurs Forum. 2007;34(4):1–8. https://doi.org/10.1188/07.ONF.xxx-xxx
-
Eghbali M, Yekaninejad M, Varaei S, Jalalinia S, Samimi M, Sa’atchi K. The effect of auricular acupressure on nausea and vomiting caused by chemotherapy among breast cancer patients. Complement Ther Clin Pract. 2016;24:189–94. https://doi.org/10.1016/j.ctcp.2016.06.006
-
Genç F, Tan M. The effect of acupressure application on chemotherapy-induced nausea, vomiting, and anxiety in patients with breast cancer. Palliative & Supportive Care. 2015;13(2):275-84. https://doi.org/10.1017/S1478951514000248
-
Genç A, Can G, Aydiner A. The efficiency of the acupressure in prevention of the chemotherapy-induced nausea and vomiting. Support Care Cancer. 2013;21(1):253-261. https://doi.org/10.1007/s00520-012-1519-3
-
Molassiotis A, Helin AM, Dabbour R, Hummerston S. The effects of P6 acupressure in the prophylaxis of chemotherapy-related nausea and vomiting in breast cancer patients. Complement Ther Med. 2007;15(1):3-12. https://doi.org/10.1016/j.ctim.2006.07.005
-
Molassiotis A, Russell W, Hughes J, Breckons M, Lloyd-Williams M, Richardson J, et al. The effectiveness and cost-effectiveness of acupressure for the control and management of chemotherapy-related acute and delayed nausea: Assessment of Nausea in Chemotherapy Research (ANCHoR), a randomised controlled trial. Health Technol Assess. 2013;17(26):1-114. https://doi.org/10.3310/hta17260
-
Molassiotis A, Russell W, Hughes J, Breckons M, Lloyd-Williams M, Richardson J, et al. The effectiveness of acupressure for the control and management of chemotherapy-related acute and delayed nausea: A Randomized clinical trial. J Pain Symptom Manage. 2014;47(1):12-25. https://doi.org/10.1016/j.jpainsymman.2013.03.007
-
Shen CH, Yang LY. The Effects of Acupressure on Meridian Energy as well as Nausea and Vomiting in Lung Cancer Patients Receiving Chemotherapy. https://doi.org/10.1177/1099800416683801
-
Shin YM, Kim TI, Shin MS, Juon HS. Effect of Acupressure on Nausea and Vomiting During Chemotherapy Cycle for Korean Postoperative Stomach Cancer Patients. Cancer Nursing. 2004;27(4):267-274. https://doi.org/10.1097/00002820-200407000-00002
-
Suh EE. The effects of P6 acupressure and nurse-provided counseling on chemotherapy-induced nausea and vomiting in patients with breast cancer. Oncol Nurs Forum. 2012;39(1):e1-9. https://doi.org/10.1188/12.ONF.E1-E9
-
Akhu-Zaheya LM, Khater WA, Lafi AY. The effectiveness of hologram bracelets in reducing chemotherapy-induced nausea and vomiting among adult patients with cancer. Cancer Nurs. 2017;40(2):E17–29. https://doi.org/10.1097/NCC.0000000000000374
-
Pearl ML, Fischer M, McCauley DL, Valea FA, Chalas E. Transcutaneous electrical nerve stimulation as an adjunct for controlling chemotherapy-induced nausea and vomiting in gynecologic oncology patients. Cancer Nurs. 1999;22(4):307–311. https://doi.org/10.1097/00002820-199908000-00008
-
Betticher DC, Delmore G, Breitenstein U, Anchisi S, Zimmerli-Schwab B, Müller A, et al. Efficacy and tolerability of two scalp cooling systems for the prevention of alopecia associated with docetaxel treatment. Support Care Cancer. 2013;21:2565–2573. https://doi.org/10.1007/s00520-013-1804-9
-
Giaccona G, Di Giulio F, Morandini MP, Calciati A. Scalp hypothermia in the prevention of doxorubicin-induced hair loss. Cancer Nurs. 1988;11(3):170-173. https://pubmed.ncbi.nlm.nih.gov/3401852/
-
Kargar M, Sarvestani RS, Khojasteh HN, Heidari MT. Efficacy of penguin cap as scalp cooling system for prevention of alopecia in patients undergoing chemotherapy. J Adv Nurs. 2011;67(11):2473–7. https://doi.org/10.1111/j.1365-2648.2011.05668.x
-
Macduff C, Mackenzie T, Hutcheon A, Melville L, Archibald H. The effectiveness of scalp cooling in preventing alopecia for patients receiving epirubicin and docetaxel. Eur J Cancer Care. 2003;12(2):154–61. https://doi.org/10.1046/j.1365-2354.2003.00382.x
-
Nangia J, Tao W, Osborne C, Niravath P, Otte K, Papish S, et al. Effect of a Scalp Cooling Device on Alopecia in Women Undergoing Chemotherapy for Breast Cancer: The SCALP Randomized Clinical Trial. JAMA. 2017;317(6):596-605. https://jamanetwork.com/journals/jama/fullarticle/2601500
-
Lemenager M, Lecomte S, Bonneterre ME, Bessa E, Dauba J, Bonneterre J. Effectiveness of cold cap in the prevention of docetaxel-induced alopecia. Eur J Cancer. 1997;33(2):297-300. https://doi.org/10.1016/S0959-8049(96)00374-7
-
Nolte S, Donnelly J, Kelly S, Conley P, Cobb R. A randomized clinical trial of a videotape intervention for women with chemotherapy-induced alopecia: a gynecologic oncology group study. Oncol Nurs Forum. 2006;33(2):305-11. https://pubmed.ncbi.nlm.nih.gov/16518446/
-
Rugo H, Klein P, Melin S, Hurvitz S, Melisko M, Moore A, et al. Association Between Use of a Scalp Cooling Device and Alopecia After Chemotherapy for Breast Cancer. JAMA. 2017;17(6):606-614https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5639721/
-
Rustøen T, Valeberg BT, Kolstad E, Wist E, Paul S, Miaskowski C. A randomized clinical trial of the efficacy of a self-care intervention to improve cancer pain management. Cancer Nurs. 2014;37(1):34–43. https://doi.org/10.1097/NCC.0b013e3182948418
-
De Wit R, Van Dam F, Zandbelt L, Van Buuren A, Van der Heijden K, Leenhouts G, et al. A Pain Education Program for chronic cancer pain patients: Follow-up results from a Randomized clinical trial. Pain. 1997;73(1):55–69. https://doi.org/10.1016/s0304-3959(97)00070-5
-
Aghabati N, Mohammadi E, Pour Esmaiel Z. The Effect of Therapeutic Touch on Pain and Fatigue of Cancer Patients Undergoing Chemotherapy. Evid Based Complement Alternat Med. 2010;7(3):375https://doi.org/10.1093/ecam/nen006
-
Miladinia M, Baraz S, Shariati A, Malehi AS. Effects of Slow-Stroke Back Massage on Symptom Cluster in Adult Patients with Acute Leukemia: Supportive Care in Cancer Nursing. Cancer Nurs. 2017;40(1):31–8. https://doi.org/10.1097/NCC.0000000000000353
-
Dhawan S, Andrews R, Kumar L, Wadhwa S, Shukla G. A Randomized clinical trial to Assess the Effectiveness of Muscle Strengthening and Balancing Exercises on Chemotherapy-Induced Peripheral Neuropathic Pain and Quality of Life Among Cancer Patients. Cancer Nurs. 2020;43(4):269–80. https://doi.org/10.1097/NCC.0000000000000693
-
Yildirim M, Gulsoy H, Batmaz M, Ozgat C, Yesilbursali G, Aydin R et al. Symptom management: The effects of self-affirmation on chemotherapy-related symptoms. Clin J Oncol Nurs. 2017;21(1):E15-22. https://doi.org/10.1188/17.CJON.E15-E22
-
Given B, Given CW, McCorkle R, Kozachik S, Cimprich B, Rahbar MH, et al. Pain and fatigue management: results of a nursing randomized clinical trial. Oncol Nurs Forum. 2002;29(6):949–56. https://doi.org/10.1188/02.ONF.949-956
-
Park R, Park C. Comparison of foot bathing and foot massage in chemotherapy-induced peripheral neuropathy. Cancer Nurs. 2015;38(3):239–47https://doi.org/10.1097/NCC.0000000000000181
-
Andersen Hammond E, Pitz M, Steinfeld K, Lambert P, Shay B. An Exploratory Randomized Trial of Physical Therapy for the Treatment of Chemotherapy-Induced Peripheral Neuropathy. Neurorehabil Neural Repair. 2020;34(3):235–46. https://doi.org/10.1177/1545968319899918
-
Zhi WI, Baser RE, Talukder D, Mei YZ, Harte SE, Bao T. Mechanistic and thermal characterization of acupuncture for chemotherapy-induced peripheral neuropathy as measured by quantitative sensory testing. Breast Cancer Res Treat. 2023;197(3):535–45. https://doi.org/10.1007/s10549-022-06846-3
-
Arslan S, Zorba Bahceli P, İlik Y, Artaç M. The preliminary effects of henna on chemotherapy-induced peripheral neuropathy in women receiving oxaliplatin-based treatment: A parallel-group, randomized, controlled pilot trial. Eur J Oncol Nurs. 2020;48. https://doi.org/10.1016/j.ejon.2020.101827
-
Greenlee H, Crew KD, Capodice J, Awad D, Buono D, Shi Z, et al. Randomized sham-controlled pilot trial of weekly electro-acupuncture for the prevention of taxane-induced peripheral neuropathy in women with early-stage breast cancer. Breast Cancer Res Treat. 2016;156(3):453–64.https://doi.org/10.1007/s10549-016-3759-2
-
Tsao Y, Creedy DK. Auricular acupressure: reducing side effects of chemotherapy in women with ovarian cancer. Supportive Care in Cancer. 2019;27(11):4155–4163.https://doi.org/10.1007/s00520-019-04682-8
-
Kuo HC, Tsao Y, Tu HY, Dai ZH, Creedy DK. Pilot Randomized clinical trial of auricular point acupressure for sleep disturbances in women with ovarian cancer. Res Nurs Health. 2018;41(5):469–79. https://doi.org/10.1002/nur.21885
-
Coleman EA, Goodwin JA, Kennedy R, Coon SK, Richards K, Enderlin C, et al. Effects of Exercise on Fatigue, Sleep, and Performance: A Randomized Trial. Oncol Nurs Forum. 2012;39(5):468-77. https://doi.org/10.1188/12.ONF.468-477
-
Yang HL, Chen XP, Lee KC, Fang FF, Chao YF. The effects of warm-water footbath on relieving fatigue and insomnia of gynecologic cancer patients on chemotherapy. Cancer Nurs. 2010;33(6):454–60. https://doi.org/10.1097/NCC.0b013e3181d761c1
-
Chuang T, Yeh M, Chung Y. A nurse facilitated mind-body interactive exercise (Chan-Chuang qigong) improves the health status of non-Hodgkin lymphoma patients receiving chemotherapy: Randomised controlled trial. Int J Nurs Stud. 2017;69:25–33. https://doi.org/10.1016/j.ijnurstu.2017.01.004
-
Yang W, Xi J, Guo L, Cao Z. Nurse-led exercise and cognitive-behavioral care against nurse-led usual care between and after chemotherapy cycles in Han Chinese women of ovarian cancer with moderate to severe levels of cancer-related fatigue: A retrospective analysis of the effectiveness. Medicine. 2021;100(44):e27317. https://doi.org/10.1097/MD.0000000000027317
-
Reich RR, Lengacher CA, Klein TW, Newton C, Shivers S, Ramesar S, et al. A Randomized clinical trial of the Effects of Mindfulness-Based Stress Reduction (MBSR[BC]) on Levels of Inflammatory Biomarkers Among Recovering Breast Cancer Survivors. Biol Res Nurs. 2017;19(4):456–64. https://doi.org/10.1177/1099800417707268
-
Yeh M, Lee T, Chen H, Chao T. The influences of Chan-Chuang qi-gong therapy on complete blood cell counts in breast cancer patients treated with chemotherapy. Cancer Nurs. 2006;29(2):149–55. https://doi.org/10.1097/00002820-200603000-00012
-
Centros para el control y la prevención de enfermedades-CDC. Medicina complementaria y alternativa. Consulta: Agosto 08, 2023. Disponible en: https://www.cdc.gov/cancer-survivors/es/patients/complementary-alternative-medicine.html?CDC_AAref_Val=https://www.cdc.gov/spanish/cancer/survivors/patients/complementary-alternative-medicine.htm
-
Instituto Nacional del cáncer. Medicina complementaria y alternativa 2015. Consulta: Agosto 08, 2023. Disponible en: https://www.cancer.gov/espanol/cancer/tratamiento/mca
-
Idoyaga Molina N, Luxardo N. Medicinas no convencionales en cáncer. Medicina (B Aires). 2005;65(5):390-394. http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S0025-76802005000500002
-
Heckroth M, Luckett RT, Moser C, Parajuli D, Abell TL. Nausea and Vomiting in 2021: A Comprehensive Update. J Clin Gastroenterol. 2021;55(4):279–99. https://doi.org/10.1097/MCG.0000000000001485
-
Turner L, Lau V, Neeson S, Davies M. International Exchange Programs: Professional Development and Benefits to Oncology Nursing Practice. Clin J Oncol Nurs. 2019;23(4):439–42. https://doi.org/10.1188/19.CJON.439-442
-
Lee A, Chan SKC, Fan LTY. Stimulation of the wrist acupuncture point PC6 for preventing postoperative nausea and vomiting. Cochrane Libr. 2015;(11). https://doi.org/10.1002/14651858.CD003281.pub4
-
Morehead A, Salmon G. Efficacy of Acupuncture/Acupressure in the Prevention and Treatment of Nausea and Vomiting Across Multiple Patient Populations: Implications for Practice. Nurs Clin North Am. 2020;55(4):571–80. https://doi.org/10.1016/j.cnur.2020.07.001
-
De Paolis G, Naccarato A, Cibelli F, D’Alete A, Mastroianni C, Surdo L, et al. The effectiveness of progressive muscle relaxation and interactive guided imagery as a pain-reducing intervention in advanced cancer patients: A multicentre randomised controlled non-pharmacological trial. Complement Ther Clin Pract. 2019;34:280–7.https://doi.org/10.1016/j.ctcp.2018.12.014
-
Wallace KG. Analysis of recent literature concerning relaxation and imagery interventions for cancer pain. Cancer Nurs. 1997;20(2):79–88. https://doi.org/10.1097/00002820-199704000-00001
-
Mora DC, Overvåg G, Jong MC, Kristoffersen AE, Stavleu DC, Liu J, et al. Complementary and alternative medicine modalities used to treat adverse effects of anti-cancer treatment among children and young adults: a systematic review and meta-analysis of Randomized clinical trials. BMC Complement Med Ther. 2022;22(1):97. https://doi.org/10.1186/s12906-022-03537-w
-
Coelho A, Parola V, Cardoso D, Bravo ME, Apóstolo J. Use of non-pharmacological interventions for comforting patients in palliative care: a scoping review. JBI Database System Rev Implement Rep. 2017;15(7):1867–904. https://doi.org/10.11124/JBISRIR-2016-003204
-
Freites-Martinez A, Shapiro J, Goldfarb S, Nangia J, Jimenez JJ, Paus R, et al. Hair disorders in patients with cancer. J Am Acad Dermatol. 2019;80(5):1179–96. https://doi.org/10.1016/j.jaad.2018.03.055
-
Mao J, Pillai G, Andrade C, Ligibel J, Basu P, Cohen L, et al. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J Clin. 2022;72(2):144–64. https://doi.org/10.3322/caac.21706