Rev Cuid. 2024; 15(1): e3624
Abstract
Introduction: Healthcare-associated infections pose a significant challenge, contributing to hospital morbidity and mortality. Objective: To describe the behavior of Healthcare Associated Infections before and during the pandemic reported to a high-complexity health institution in Colombia. Material and Methods: In our retrospective observational study on Healthcare-Associated Infections (HAIs), we analyzed data from all in-patients diagnosed with HAIs between 2018 and 2020. This included clinical, demographic, microbiological, and microbial susceptibility information collected from the Committee on Nosocomial Infections' prospective database. Data from 391 isolates were obtained using Whonet software for antimicrobial resistance surveillance. Results: We found 504 cases of HAIs (2018-2020) with an overall in-hospital infection rate of 2.55/1000 patient-days. The median age for pediatric patients was 5 years, and for adults, 56 years, with 57% male. The leading admission diagnoses were oncologic disease complications (31%). Bacteremia had a 30-day mortality rate of 13%, predominantly catheter-associated (37%). Gram-negative bacilli, notably Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa, represented 58% cases of HAI. Discussion: The critical need for specific interventions and antimicrobial management to control HAIs, especially given the challenges posed by the COVID-19 pandemic, is highlighted. Conclusions: This is the first report on HAIs incidence at a tertiary hospital in Bucaramanga, Santander (Colombia). Bacteremia was predominant; 75% of HAIs patients had comorbidities. Gram-negative bacilli prevailed; a notable rise in ICU respiratory infections occurred during the 2020 COVID-19 pandemic. Resistance to cephalosporins and carbapenems was prevalent.
Key Words: Healthcare Associated Infection; Antibiotic Resistance; Hospital Infection Control Services; COVID-19.
Resumen
Introducción: Las infecciones asociadas a la atención en salud (IAAS) representan un reto porque estas contribuyen a la morbilidad y mortalidad hospitalaria. Objetivo: Describir el comportamiento de las IAAS antes y durante la pandemia, las cuales fueron reportadas a una institución de salud de alta complejidad en Colombia. Materiales y métodos: En nuestro estudio observacional retrospectivo de las IAAS, analizamos los datos de todos los pacientes hospitalizados que fueron diagnosticados con una IAAS entre 2018 y 2020. Esto incluyó información clínica, demográfica, microbiológica y de susceptibilidad microbiana recabada de la base de datos prospectiva del Comité de Infecciones Nosocomiales. Los datos de 391 aislamientos se obtuvieron utilizando el programa informático Whonet para la vigilancia de la resistencia a los antimicrobianos. Resultados: Encontramos 504 casos de IAAS entre 2018 y 2020 con una tasa global de infección intrahospitalaria de 2,55/1000 pacientes al día. La mediana de edad de los pacientes pediátricos fue de 5 años y la de los adultos de 56 y el 57% de ellos eran varones. Los principales diagnósticos de ingreso fueron complicaciones oncológicas (31%). La bacteriemia tuvo una tasa de mortalidad a los 30 días del 13%, predominantemente asociada al uso de catéter (37%). Los bacilos gramnegativos, sobre todo Klebsiella pneumoniae, Escherichia coli y Pseudomonas aeruginosa, representaron el 58% de los casos de IAAS. Discusión: Se destaca la necesidad crítica de contar con intervenciones específicas y de gestión antimicrobiana para controlar las IAAS, especialmente teniendo en cuenta los retos que planteó la pandemia de Covid-19. Conclusiones: Este es el primer informe sobre la incidencia de las IAAS en un hospital terciario de Bucaramanga, Santander (Colombia). La bacteriemia predominó y 75% de los pacientes con IAAS presentaban comorbilidades. Predominaron los bacilos gramnegativos y se produjo un notable aumento de las infecciones respiratorias en las UCI durante la pandemia Covid-19 de 2020. Fue prevalente la resistencia a las cefalosporinas y a los carbapenémicos.
Palabras Clave: Infecciones Asociadas con el Sistema de Salud; Resistencia a Antibióticos; Servicios de Control de Infección Hospitalaria; COVID-19.
Resumo
Introdução: As infeções associadas aos cuidados de saúde representam um desafio significativo, contribuindo para a morbilidade e mortalidade hospitalar. Objetivo: Descrever o comportamento das Infecções Relacionadas à Assistência à Saúde antes e durante a pandemia notificadas a uma instituição de saúde de alta complexidade na Colômbia. Material e Métodos: Em nosso estudo observacional retrospectivo sobre Infecções Relacionadas à Assistência à Saúde (IRAS), analisamos dados de todos os pacientes internados com diagnóstico de IRAS entre 2018 e 2020. Isso incluiu informações clínicas, demográficas, microbiológicas e de suscetibilidade microbiana coletadas do Comitê no banco de dados prospectivo de infecções hospitalares. Os dados de 391 isolados foram obtidos utilizando o software Whonet para vigilância da resistência antimicrobiana. Resultados: Foram encontrados 504 casos de IRAS (2018-2020) com taxa global de infecção hospitalar de 2,55/1.000 pacientes-dia. A idade média para pacientes pediátricos foi de 5 anos, para adultos 56 anos, sendo 57% do sexo masculino. Os principais diagnósticos de admissão foram complicações de doenças oncológicas (31%). A bacteremia teve uma taxa de mortalidade em 30 dias de 13%, predominantemente associada ao cateter (37%). Bacilos Gram-negativos, notadamente Klebsiella pneumoniae, Escherichia coli e Pseudomonas aeruginosa, representaram 58% dos casos de IRAS. Discussão: É destacada a necessidade crítica de intervenções específicas e gestão antimicrobiana para controlar as IACS, especialmente tendo em conta os desafios colocados pela pandemia da COVID-19. Conclusões: Este é o primeiro relatório sobre a incidência de IRAS em um hospital terciário em Bucaramanga, Santander (Colômbia). A bacteremia foi predominante; 75% dos pacientes com IRAS apresentavam comorbidades. Prevaleceram bacilos Gram-negativos; um aumento notável nas infecções respiratórias em UTI ocorreu durante a pandemia de COVID-19 de 2020. A resistência à cefalosporina e aos carbapenêmicos foi prevalente.
Palavras-Chave: Infecções Associadas ao Sistema de Saúde; Resistência a Antibióticos; Serviços de Controle de Infecção Hospitalar; COVID-19.
Introduction
Healthcare-Associated Infections (HAIs) continue to contribute substantially to hospital morbidity and mortality1. Especially when associated with antibiotic-resistant microorganisms. Prevalence studies in the United States (US) suggest that 30% of HAIs occur in intensive care units (ICUs)2,3. The increase in the incidence of HAIs is largely due to the multiple interventions of modern medicine, by using new medical devices, organ transplants, broad-spectrum antibiotics, and long hospital stays, among others4-7 These HAIs have become one of the most important challenges in medical practice, as pointed out by the World Health Organization (WHO)8.
It is estimated that the prevalence of HAIs in developing countries is significantly higher compared to developed countries, as described in a recent meta-analysis where regions such as North America and Europe report a prevalence between 4 to 7 cases per 100 hospitalized patients, contrary to reports in Latin America, Africa, and Asia where the prevalence can reach up to 16 cases per 100 hospitalized patients9. This situation is even more worrying when describing the incidence of HAIs in Intensive Care Units (ICU), where countries such as Brazil reach between 14-62 cases per 1000 patients compared to the United States which reports between 6-9 cases per 1000 patients4,10. This data coincides with estimates that between 20-30% of all HAIs in the hospital are acquired in ICUs with the presence of Multi-Drug Resistant Organisms (MDRO)10,11.
In Colombia, Enterobacterales (Escherichia coli, Klebsiella spp, and Enterobacter spp) rank highest in the epidemiology of HAIs (Healthcare-Associated Infections)12. These bacteria can develop resistance to various carbapenems through the production of hydrolytic enzymes such as Extended-Spectrum Beta-Lactamases (ESBLs), AmpC cephalosporinases, carbapenemases, or mutations in outer membrane proteins13. Carbapenemases were first reported in Colombia in 2006, and since then, various studies have demonstrated a significant increase in bacterial resistance. For instance, cephalosporins showed a resistance rate of 21.70% between 1997-2000, and subsequently, between 2013-2016, an increase of 63.00% was observed in the analyzed isolates14.
During the SARS-CoV-2 pandemic, hospital institutions faced immense pressure, demanding significant economic and human efforts, especially from healthcare workers. It required relocation and training in new hospital areas divided to treat both COVID and non-COVID patients, particularly in ICUs15.
Antimicrobial resistance during the SARS-CoV-2 pandemic, along with the increased use of antibiotics, raised the likelihood of developing a secondary HAI (Healthcare-Associated Infection) due to COVID-1916,17. The reason why active epidemiological surveillance of HAIs within healthcare institutions is necessary is to understand which microorganisms are most common and to observe changes in antimicrobial susceptibility profiles that may prevent future outbreaks within healthcare facilities18. The objective of this study is to describe the behavior of Healthcare Associated Infections before and during the pandemic reported to a high-complexity health institution in Colombia, between the years 2018 and 2020.
Materials and Methods
Study design
This was a retrospective cohort study aimed at describing the changes in HAIs in the high complexity institution before and during the COVID-19 pandemic. The study was conducted over three years from January 1, 2018 to December 31, 2020, in which 244,889 hospital admissions were reported. We analyzed all patients admitted to the hospital from January 1, 2018 to March 29, 2020, the date on which the first SARS-CoV-2 positive patient was admitted to our institution (168,034 hospital admissions) and compared it with patients admitted during the COVID-19 pandemic that included the period of time between March 30, 2020 to December 31, 2020 (76,885 hospital admissions).
Clinical setting and data collection
The study took place in a Joint Commission-accredited 286-bed fourth level care institution in Bucaramanga, Santander, Colombia. The hospital has a 187-bed capacity in adult general wards and 99 in adult intensive care unit (ICU). Case definition for HAI followed the Centers for Disease Control HAI criteria, which defines HAI as a localized or systemic condition resulting from an adverse reaction to the presence of an infectious agent(s) or its toxin(s), after the 3rd hospital day (day of hospital admission is day 1). There must be no evidence that the infection was present or incubating at the time of admission hospital19.
All patients with a confirmed diagnosis of HAIs (n=504) were included in the analysis. Clinical and demographic data were collected from the hospital's electronic medical records, including age, sex, date of admission, type of admission, discharge date, status of the patient at discharge, pre-existing comorbidities; antimicrobial therapy; diagnosed HAIs and microbiological cultures performed, locating the site of infection, date of HAI onset and microbiological confirmation. The analysis for site of infection was performed according to the following groups, bloodstream, includes bacteremia associated with central venous catheter, Hickman Broviac catheter, Mahurkar catheter, peripherally inserted central catheter, fungemia and bacteremia associated with mucosal barrier injury; urinary tract includes urinary tract and bladder catheter-associated infections; surgical site includes superficial and deep surgical site infection, organ space surgical site infection, and soft tissue infection; respiratory tract includes tracheobronchitis associated with mechanical ventilation, cases of pneumonia, tracheitis, aspiration pneumonia, and aspiration tracheobronchitis. For COVID-19 patients, laboratory confirmation of SARS-CoV-2 was defined as a positive result of real-time reverse transcriptase-polymerase chain reaction assay of nasal and pharyngeal swabs20.
Microbiological identification
For each case of HAI, the type of infection, pathogen identification and microbiological susceptibility were obtained from the medical record and WHONET Software (version 5.6), used for surveillance of antimicrobial susceptibility21. Identification of microbial species and antimicrobial resistance patterns were determined using the VITEK-2 Compact system (BioMerieux SA, France). The following quality control strains were included: Staphylococcus aureus [American Type Culture Collection (ATCC) 25923], Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 700603; BAA1705) and Pseudomonas aeruginosa (ATCC 27853).
Data Set
The validated information was stored in GitLab22.
Statistical analysis
Clinical and microbiological data were collected and by the principal investigator and data analyst. For statistical analyses, a descriptive analysis was initially performed, where categorical values are presented as proportions and continuous variables as means and standard deviation (SD). Otherwise, these variables were described as medians and their interquartile range (IR). The Chi2 test was used to determine if there were statistically significant differences between the categorical variables and the student’s t-test or the Mann-Whitney U-test for the continuous variables, according to their distribution. All data were analyzed in Stata statistical software version 15.0 (Stata Corporation, College Station, TX). Statistical significance was defined as a p-value <0.05.
Ethics considerations
This study was approved by the ethics committee of the Fundación Cardiovascular de Colombia (Acta # 525 of December 15, 2020).
Results
A total of 244,889 hospital admissions comprising 168,034 hospital admissions recorded from January 1, 2018, to March 29, 2020 (referred to as the 2018/2019 cohort) and 76,855 hospital admissions from March 30, 2020, to December 31, 2020 (described as the 2020 cohort). Among all the admissions, 504 cases of HAI were reported, with an average of 166.52 cases per year in the 2-year period prior to COVID-19 (total of 333 cases, rate of 0.13% per hospital admission), and 171 cases reported after the first case of SARS-CoV-2 infection was confirmed in the institution on March 29, 2020 (rate of 0.67% per hospital admission). A detailed description of the patient demographics is provided in (Table 1).
Table 1. Characteristics of the patients (n=504)
X
Table 1. Characteristics of the patients (n=504)
|
2018/2019 (year) |
2020 (year) |
p-value |
| Patients |
333 |
171 |
- |
| Observation time, person-days |
114,776 |
83,087 |
- |
| Gender (male) |
54.00 (180) |
65.00 (111) |
0.0181‡ |
| Age, years (mean ± SD) |
46.30 ± 28.0 |
48.60 ± 23.8 |
0.3594¥ |
| Pediatric patients (<18 years) |
25.22 (84) |
16.95 (29) |
0.0345‡ |
| Age of pediatric patients, (mean ± SD) |
4.40 ± 5.10 |
7.42 ± 6.20 |
0.3594¥ |
| Age of adult patients, (mean ± SD) |
56.80 ± 17.7 |
57.0 ± 15.9 |
0.9012¥ |
| Coexisting conditions %(n) |
|
|
|
| Coronary heart disease |
10.81 (36) |
11.11 (19) |
0.9186‡ |
| Chronic lung disease |
10.81 (36) |
11.11 (19) |
0.9186‡ |
| Autoimmune disease |
3.60 (12) |
5.26 (9) |
0.3774‡ |
| Endocrine disease |
5.10 (17) |
5.84 (10) |
0.7258‡ |
| Chronic renal disease |
8.70 (29) |
21.63 (37) |
< 0.0001‡ |
| Hypertension |
34.53 (115) |
34.50 (59) |
- |
| Diabetes mellitus |
12.91 (43) |
25.73 (44) |
0.1362‡ |
| Obesity |
3.00 (10) |
14.61 (25) |
< 0.0001‡ |
| Psychiatric disorder |
19.82 (66) |
15.78 (27) |
0.0540‡ |
| Cancer |
45.64 (152) |
19.88 (34) |
<0.00001‡ |
| Genetic disease |
2.10 (7) |
7.01 (12) |
0.0061‡ |
| Central nervous system disorders |
18.31 (61) |
39.76 (68) |
< 0.0001‡ |
| Immunosuppression |
28.82 (96) |
35.67 (61) |
< 0.1188‡ |
| Infection site. %(n) |
|
|
|
| Bloodstream |
42.94 (143) |
47.36 (81) |
0.3470‡ |
| Urinary tract |
16.51 (55) |
23.97 (41) |
0.0452‡ |
| Surgical site |
27.32 (91) |
12.86 (22) |
0.0002‡ |
| Respiratory tract |
13.21 (44) |
15.78 (27) |
0.4449‡ |
| Length of hospital stay (days), median (IQR) |
77 (18-95) |
33 (17-50) |
0.4278¥ |
| Admission to ICU |
36.33 (121) |
52.63 (90) |
0.0004‡ |
| Central venous catheter |
35.13 (117) |
38.59 (66) |
0.5046‡ |
| Urinary catheter |
19.81 (66) |
25.14 (43) |
0.1113‡ |
| Invasive ventilation |
15.91 (53) |
20.46 (35) |
0.2025‡ |
| Positive RT-PCR for SARS-CoV-2 |
N/A |
33.91 (58) |
- |
| Mortality. %(n) |
9.90 (33) |
28.07 (48) |
< 0.0001‡ |
| Mortality rate (95% CI) per 1000 patient-days |
2.90 (2.01- 3.99) |
5.77 (4.30- 7.59) |
- |
| 30-day mortality in patients with bacteremia. %(n) |
5.10 (17) |
11.11 (19) |
0.0133‡ |
| Re-admission. %(n) |
16.81 (56) |
17.54 (30) |
0.8433‡ |
Notes: %: percentage; IQR: interquartile range; SD: standard deviation; ‡p value determined by Chi2 test; ¥ p value determined by Student’s t test.
In both cohorts, most of the patients were men, especially in the 2020 cohort (65.00%). The mean age was similar between cohorts (46.3 vs 48.6 years), but the proportion of pediatric patients was higher in the 2018/2019 cohort (25.22%). In the 2018/2019, cancer, immunodeficiencies, and hypertension were the most common coexisting conditions, compared to the 2020 cohort where central nervous system (CNS) pathologies, immunodeficiencies, and hypertension were the most common. In the 2020, there was a 21.45%, 12.93%, and 11.61% increase in patients with HAIs that also presented with CNS pathologies, chronic renal disease, and obesity, respectively, compared to the 2018/2019 cohort (p < 0.0001). Conversely, there was a 25.76% reduction in the 2020 cohort, compared to the 2018/2019 cohort, among patients with cancer and HAIs. In both cohorts, the more commonly identified site of infection was blood. However, in the 2020 cohort there was a significant reduction in surgical site infections (p = 0.0002), likely due to the decreased surgical procedures performed in our institution during the early stages of the COVID-19 pandemic. While the median length of stay decreased between the 2018/2019 cohort (31 days) and the 2020 cohort (28 days), the proportion of patients admitted to the ICU increased by 16.30%. As for invasive devices, patients hospitalized in 2020 were more likely to have urinary catheters and respiratory support through mechanical ventilation. Overall mortality was significantly higher in the 2020 cohort (28.00%), with a mortality rate of 5.77 (95% CI 4.3-7.6) per 1,000 patients-days. By contrast, the mortality rate in 2018/2019 was 2.9 (95% CI 2.0-4.0) per 1,000 patients-days. Rates of readmission were similar between both cohorts (16.81% vs 17.54%, p = 0.84). Within the cases observed in the 2020 cohort, 58 HAIs (33.91%) corresponded to patients who tested positive for SARS-CoV-2. These patients were more likely to be male, older (median age 60.7 years) and obese (Supplementary Table 1). A higher proportion of the patients with both SARS-CoV-2 infection and HAIs died (63.70%) compared to those without SARS-CoV-2 (12.52%). However, the mortality rate was similar between subgroups.
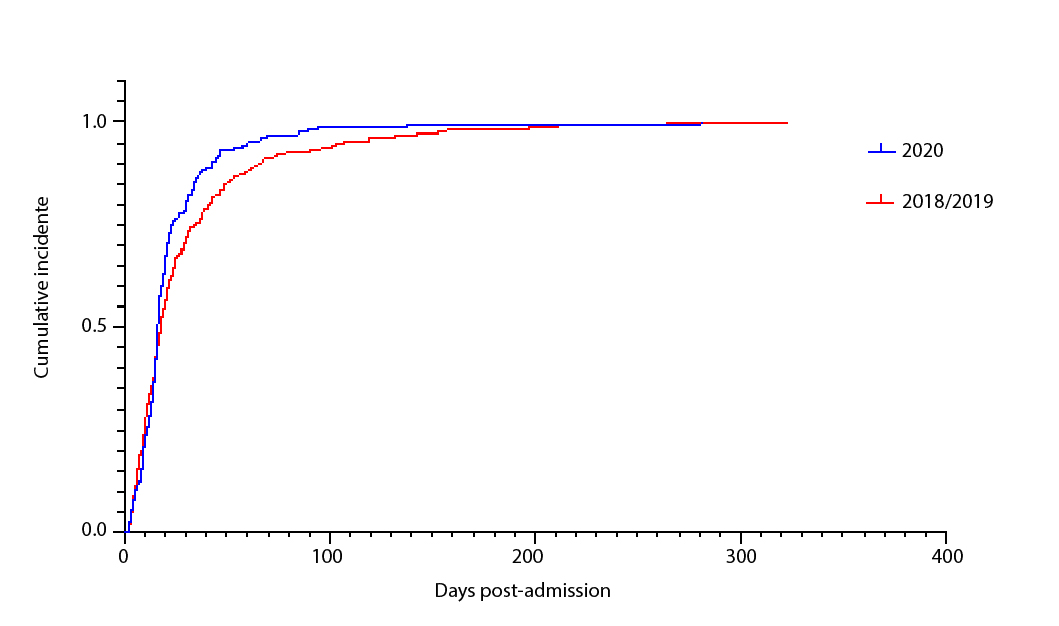
While the overall proportion of patients with HAIs was similar between cohorts, patients with HAIs in the 2020 cohort were more likely to become infected earlier after being admitted to the hospital (Figure 1).
The frequency of HAIs by site of infection and hospitalization service was similar in most cases, except for bloodstream infections, urinary tract infections, and respiratory tract infections of patients in intensive care unit, which increased to >100% for respiratory infections in the 2020 cohort, according to the significant increase in the 30.00% available beds ICU in the number of COVID-19 cases seen (Figure 2, Supplementary Table 2).
Most infections were monomicrobial 486 (96.42%), only 27 infections were polymicrobial, of which 17 were gram-negative pathogen and 8 with one gram-positive and one gram-negative organism. There was one mixed infection with two fungi and another infection with gram-negative organism and one fungus. In both cohorts, the most common isolations were Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa (Table 2). Overall, Enterobacterales were the causative organisms in 52.54% of HAIs in the 2018/2019 cohort and 50.28% of HAIs in the 2020 cohort. In the 2020 cohort, there was a 4.65% increase in cases of Pseudomonas aeruginosa compared to the prior years. Similarly, infections by Candida spp. Increased by 5.78%. A detailed distribution of causative organism per site of infection is provided in (supplementary Figure 1). While Enterobacterales were the most common organism isolated from bloodstream, urinary tract and surgical site HAIs, Pseudomonas aeruginosa was the most common pathogen in respiratory tract HAIs
Table 2. Microorganisms isolated.
X
Table 2. Microorganisms isolated.
| Microorganism |
2018/2019 cohort |
2020 cohort |
% change |
| %(n) | %(n) |
| Klebsiella pneumoniae |
24.62 (82) |
21.64 (37) |
-2.99 |
| Escherichia coli |
13.51 (45) |
15.20 (26) |
1.69 |
| Pseudomonas aeruginosa |
12.31 (41) |
16.96 (29) |
4.65 |
| Staphylococcus aureus |
6.31 (21) |
8.77 (15) |
2.46 |
| Enterobacter cloacae |
5.41 (18) |
3.51 (6) |
-1.9 |
| Proteus mirabilis |
4.20 (14) |
1.75 (3) |
-2.45 |
| Acinetobacter baumannii |
2.10 (7) |
1.75 (3) |
-0.35 |
| Staphylococcus epidermidis |
1.80 (6) |
2.34 (4) |
0.54 |
| Pseudomonas putida |
1.50 (5) |
1.17 (2) |
-0.33 |
| Morganella morgannii |
1.50 (5) |
0.58 (1) |
-0.92 |
| Aeromonas hydrophila |
1.20 (4) |
0.58 (1) |
-0.62 |
| Serratia marcescens |
1.20 (4) |
1.75 (3) |
0.55 |
| Enterobacter aerogenes |
1.20 (4) |
1.17 (2) |
-0.03 |
| Streptococcus mitis |
0.90 (3) |
0.00 (0) |
-0.90 |
| Streptococcus agalactiae |
0.60 (2) |
0.00 (0) |
-0.60 |
| Stenotrophomonas maltophilia |
0.90 (3) |
0.58 (1) |
-0.32 |
| Candida albicans |
0.60 (2) |
3.51 (6) |
2.91 |
| Candida tropicalis |
0.30 (1) |
2.92 (5) |
2.62 |
| Candida parapsilosis |
0.90 (3) |
1.17 (2) |
0.27 |
| Candida glabrata |
0.30 (1) |
0.00 (0) |
-0.30 |
| Candida haemulonii |
0.00 (0) |
0.58 (1) |
0.58 |
| Candida krusei |
0.30 (1) |
0.00 (0) |
-0.30 |
| Enterococcus faecalis |
0.60 (2) |
3.51 (6) |
2.91 |
| Enterococcus faecium |
0.00 (0) |
1.17 (2) |
1.17 |
| Proteus penneri |
0.60 (2) |
0.00 (0) |
-0.60 |
| Klebsiella oxytoca |
0.30 (1) |
0.00 (0) |
-0.30 |
| Pantoea spp |
0.90 (3) |
0.00 (0) |
-0.90 |
| Staphylococcus capitis |
0.30 (1) |
0.00 (0) |
-0.30 |
| Staphylococcus warneri |
0.30 (1) |
0.00 (0) |
-0.30 |
| Staphylococcus hominis |
0.00 (0) |
0.58 (1) |
0.58 |
| Streptococcus anginosus |
0.00 (0) |
0.58 (1) |
0.58 |
| Streptococcus sanguinis |
0.00 (0) |
0.58 (1) |
0.58 |
| Streptococcus pseudoporcinus |
0.00 (0) |
0.58 (1) |
0.58 |
| Aeromonas sobria |
0.30 (1) |
0.00 (0) |
-0.30 |
| Chryseobacterium indologenes |
0.30 (1) |
0.00 (0) |
-0.30 |
| Salmonella enterica arizonae |
0.30 (1) |
0.00 (0) |
-0.30 |
| Haemophilus influenzae |
0.30 (1) |
0.00 (0) |
-0.30 |
| Achromobacter xylosoxidans |
0.30 (1) |
0.00 (0) |
-0.30 |
| Myroides spp |
0.30 (1) |
0.00 (0) |
-0.30 |
| Listeria monocytogenes |
0.30 (1) |
0.00 (0) |
-0.30 |
| Burkholderia cepacia |
0.00 (0) |
0.58 (1) |
0.58 |
| Citrobacter koseri |
0.00 (0) |
0.58 (1) |
0.58 |
| Citrobacter youngae |
0.00 (0) |
0.58 (1) |
0.58 |
| Cupriavidus pauculus |
0.00 (0) |
0.58 (1) |
0.58 |
| Negative |
1.80 (6) |
0.58 (1) |
-1.22 |
| Without germ |
11.41 (38) |
4.09 (7) |
-7.32 |
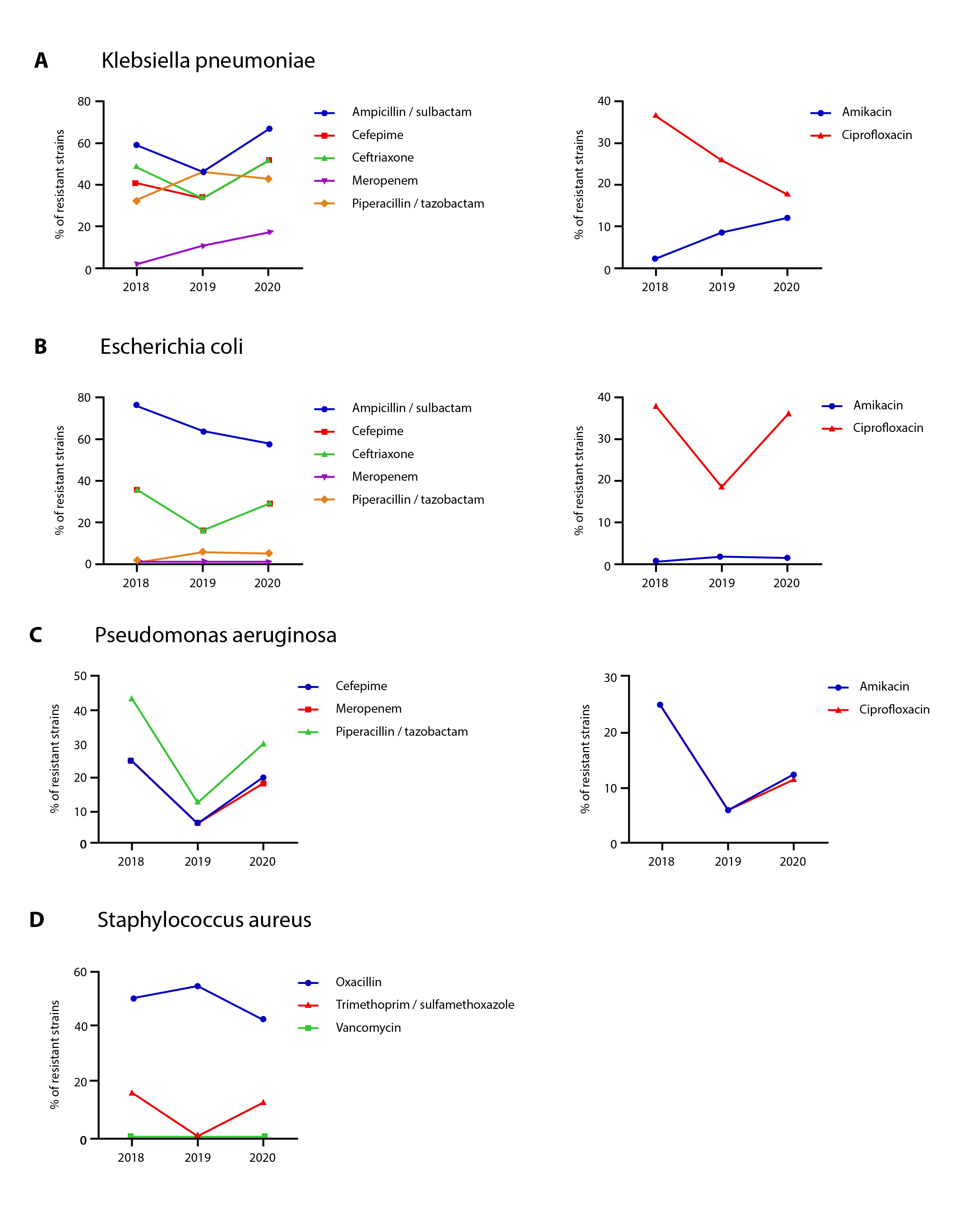
Given that Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus represent 56.75% of all cases identified as HAIs among the patients included in this study, we characterized the antimicrobial susceptibility patterns from the isolates obtained between 2018 and 2020 (Figure 3). During this period the percentage of extended spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae remained at approximately 37.00% and 45.00%, respectively. A progressive increase in resistance to meropenem was observed in Klebsiella pneumoniae, from 1.70% in 2018 to 17.00% in 2020.
Bacteremia was the most common type of HAI. In the univariate analysis (see Table 3), statistical differences were observed between the living and dead of those who had had a previous infectious disease compared to those who had not had previous infections. In addition, patients diagnosed with SARS-CoV-2 showed statistical differences in mortality and hospital stay was short for patients who died of bacteremia and pediatric population.
Table 3. Univariate analysis 30-day mortality (224 bacteremia events)
X
Table 3. Univariate analysis 30-day mortality (224 bacteremia events)
| Variables |
Alive (n=193) |
Dead (n=31) |
p-value |
| %(n) | %(n) |
| Years |
|
|
0.080† |
| 2018 |
15.54 (30) |
22.58 (7) |
|
| 2019 |
36.26 (70) |
16.12 (5) |
|
| 2020 |
48.18 (93) |
61.29 (19) |
|
| Gender |
|
|
0.815‡ |
| Female |
40.93 (79) |
38.70 (12) |
|
| Masculine |
59.06 (114) |
61.29 (19) |
|
| Population Group |
|
|
0.012‡ |
| Pediatrics |
35.75 (69) |
12.90 (4) |
|
| Adult |
64.24 (124) |
87.09 (27) |
|
| Age pediatric group, years (mean ± SD) |
5.40 ± 3.80 |
1.90 ± 1.03 |
0.9205¥ |
| Age adult group, years (mean ± SD) |
52.70 ± 24.50 |
61.50 ± 30.50 |
0.8320¥ |
| Comorbidities in adult |
(n=124) |
(n=27) |
|
| Oncologic disease |
51.61 (64) |
44.44 (12) |
0.6436‡ |
| Coronary artery disease |
14.51 (18) |
18.51 (5) |
0.070‡ |
| Chronic lung disease |
12.90 (16) |
11.11 (3) |
0.510‡ |
| Hormonal disease |
4.83 (6) |
7.40 (2) |
0.169‡ |
| Chronic renal disease |
16.12 (20) |
18.51 (5) |
0.834‡ |
| Hypertension |
41.93 (52) |
29.62 (8) |
0.657‡ |
| Diabetes mellitus |
20.16 (25) |
22.22 (6) |
0.275‡ |
| Obesity |
10.48 (13) |
11.11 (3) |
0.529‡ |
| Psychiatric disorder |
25.80 (32) |
40.74 (11) |
0.066‡ |
| Comorbidities in Pediatrics |
(n=69) |
(n=4) |
|
| Oncologic Disease |
47.82 (33) |
75.00 (3) |
0.111‡ |
| Gastrointestinal Disease |
36.23 (25) |
25.00 (1) |
0.996‡ |
| Genetic Disease |
15.94 (11) |
25.00 (1) |
0.083‡ |
| Cardiovascular Disease |
4.34 (3) |
0.00 (0) |
- |
| Previous infectious diseases |
14.49 (10) |
25.00 (1) |
0.063‡ |
| Immune suppression all ages |
52.84 (102) |
41.93 (13) |
0.259‡ |
| Diagnostic SARS-CoV 2, year 2020 (n: 112) |
11 (4.74) |
10 (27.00) |
<0.0001‡ |
| Length of hospital stay, days (mean ± SD) |
74.8 ± 85.50 |
31.8 ± 23.00 |
0.0002¥ |
| Adequate empiric therapy |
68.39 (132) |
55.88 (19) |
0.4335‡ |
SD: Standard Deviation; Hospital stays; was defined as the total number of days the patient was in the hospital until discharge; Immune suppression: Neutropenia, chemotherapy, malnutrition, and HIV/AIDS. Psychiatric disorder: Epilepsy, delirium, depression, schizophrenia, bipolar affective disorder, acute psychotic, and schizophrenia. †: determined by Fischer’s exact test ‡: p value determined by Chi2 test; ¥: p value determined by Student’s t test.
Discussion
In this study, we describe for the first time the overall incidence rate of healthcare-associated infections of HAI is 2.55 patients per 1,000 days. This is the first report of the incidence rate of HAI in a third level hospital in the city of Bucaramanga, Santander (Colombia). In Latin America there is little data on the burden of HAI. However, some countries have made progress in the characterization of this problem, as described in the study of Prevalence of Adverse Events in Latin American Hospitals - IBEAS in which nosocomial infections or currently known as HAI was the most frequent event with 37.00% in agreement, the result for Colombia occupied the first place, followed by other events related to procedures and care23.
Our study revealed that bloodstream infections were the most frequent, accounting for 45.05% of the HAIs identified during the last 3 years; we observed that this frequency increases significantly in patients with immunosuppression or oncologic disease. As reported by other studies in Latin America24. The dynamics of nosocomial infection sites may change according to the use of new medical devices and the implementation of immunosuppressive therapies implemented by each hospital institution25.
The most common microorganisms for the different types of infections were Gram-negative bacilli (Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa). In the case of Gram-positives it was Staphylococcus aureus. Similar data have been reported from other institutions of equal complexity where Gram-negative bacilli are the most common microorganisms associated with nosocomial infections26-28.
Another important finding was that 75.20% of patients with HAI had some comorbidities: arterial hypertension 34.50%, psychiatric disease 17.79%, diabetes 19.32%, chronic renal disease 15.16%, chronic pulmonary disease and coronary disease in 10.96% of the cases analyzed. Comorbidities being a considerable risk factor, which can contribute to long hospital stays, generating a negative impact on healthcare systems29.
As reported by several studies, bacteremia is associated with high mortality. In fact, (13/74) 18.00%, several studies have demonstrated similarly dismal outcomes in immunosuppressed or critically ill patients who develop sepsis, (1/14) including liver transplant patients with gram-negative sepsis30,31.
A comparison of antimicrobial resistance over time showed a significant increase in resistance to cephalosporins from 22.00% to 47.00% for Enterobacteriaceae. On the contrary, resistance to ciprofloxacin remained between 16% and 20% during the last 3 years; as for piperacillin tazobactam, there were no significant differences with a resistance between 7% and 8%. Contrary to other studies where the highest bacterial resistance is associated with cephalosporins and carbapenems32. The number of Pseudomonas aeruginosa isolates in HAI increased from 7 reported for 2018 to 43 by 2020, this significant increase may be attributed possible outbreaks that occurred in the ICU, increased use of medical devices in patients with COVID-19 among others.
The multidrug-resistant (MDR) organisms comprised 15.10% of the total isolates, including BLEE, CRE and MDR Pseudomonas MDR infections. Overall, a significant increase in reported BLEE was observed by 2020. This can be attributed to the expansion of the hospital capacity installed by COVID-19, the reconversion of services was carried out, increasing the number of intensive care beds from 21 beds to 51 beds for adults and temporarily opening in the Special Registry of Health Providers - REPS, 17 beds for adult intensive care and 18 beds for adult intermediate care; with the consequent growth in human talent, equipment and biomedical devices.
The empiric antibiotic most used in the health institution was piperacillin/tazobactam, used in 40.00% of patients with HAIs; no significant increase in resistance to this type of antibiotic was observed in our analysis. Similar data have been reported by other hospital institutions31. During the development of this study, information from both pediatric and adult populations was consolidated to perform a comprehensive and detailed analysis of all HAIs detected during the last 3 years in our institution.
It is important to mention that during the hospital stay, the causative agent was identified for 90% of all the detected HAIs together with the antimicrobial susceptibility profile, obtaining valuable information that allowed the treating physician to implement the appropriate antibiotic therapy. However, it is important to point out that a small percentage of the cultures performed could not identify the causal agent of the infection, a situation that may be associated with the fact that the high complexity institution is a reference health institution where a high number of patients are referred by other health institutions of lower complexity where patients have received empirical antibiotic treatment, which in some cases does not allow isolating and identifying the microorganism that is causing the infection for 100% of the cultures performed in the institution.
Urinary tract infections accounted for 20.00% to 56.10% of HAIs in ICU, general hospitalization, and oncology services, 46.05% of HAIs being associated with bladder catheter use, making bladder catheter use a risk factor, as described in other studies. Recent studies have shown that the inadequate use of antibiotic prophylaxis in urinary tract infections outweighs the benefits and contributes to the increase of bacterial resistance33,34, for this reason it is only recommended to use antibiotics in symptomatic infections in catheterized patients and not to use prophylaxis after catheter change in the absence of symptoms of infection35.
Among the difficulties and limitations of the study, some stand out, such as the collection of all the relevant clinical information for the 504 patients with HAIs. A possible poor quality of some of the data collected in the clinical history may underestimate important information for the analysis. However, all the information collected was done together with the medical team to clarify any doubts or uncertainties that arose in the collection of these data.
Hospital-acquired infections contribute to prolonged hospital stays and present a substantial economic burden to healthcare systems. Studies show that middle-income countries experience a higher burden of HAIs compared to developed countries, so implementing measures to prevent HAIs in middle-income countries appears to be a high-value information resource that can contribute to improved HAI control and surveillance. In addition, it can contribute to improve the standardization of reporting for the control of these infections36-38.
It is important to highlight the work performed by the Hospital Infection Committee, which collects, updates, and constantly informs all health personnel about the behavior of HAIs in this hospital. Simultaneously, it takes the measures required for any area of the hospital and highlights the importance of prevention and surveillance of these infections in the health system.
HAIs are not exclusive to any one hospital area, but some factors that contribute to the risk of contracting them have been described, whether due to long hospital stays, immunosuppression, surgical interventions, or the use of invasive medical devices, among others. For this reason, it is recommended that antimicrobial stewardship programs be implemented to effectively reduce the inappropriate use of antibiotics, especially in ICUs with the highest number of MDR organisms39,40.
Conclusions
In conclusion, bacteremia was the most frequent infection in the total number of HAIs. The most common microorganisms were Gram-negative bacteria; no differences were found between the type of infection or hospital area analyzed. Resistance to piperacillin/tazobactam increased substantially in the last year for both Enterobacterales and Pseudomonas, as did oxacillin resistance for Staphylococcus aureus. Despite the increase in antimicrobial resistance, most patients received adequate empirical antimicrobial therapy, a significant increase in the number of respiratory infections identified in the ICU was observed for the 2020 cohort (during COVID-19), due to the high number of patients treated in the hospital for SARS-CoV-2.
It is suggested that empirical treatment regimens be based on specific data from epidemiological surveillance, analysis, and interpretation of HAIs in each hospital.
It is necessary to conduct research that allows defining the cost-benefit relationship for the prevention and control of HAIs, especially evaluating the long-term impact of biosafety protocols employed during the pandemic and the elevated use of certain broad-spectrum antibiotics in patients who presented COVID-19-related complications, and their relationship with the epidemiology of HAIs.
Conflict of interest: All authors report no conflicts of interest relevant to this article.
Financing: No financial support was provided relevant to this article.
Acknowledgment: To the Committee of Hospital Infections of the high complexity institution. To the collaborators of the Microbiology Laboratory.
References
X
Referencias
Prevention of Hospital Acquired Infections: A practical Guide, G Ducel, J Gabry, L Nicolle. 2nd edition, 2002, Published by World Health Organization WHO/CDS/CSR/EPH/, Geneva; 2002.
X
Referencias
Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N Engl J Med. 2014;370(13):1198–208. https://doi.org/10.1056/NEJMoa1306801
X
Referencias
Myny D, Depuydt P, Colardyn F, Blot S. Ventilator-associated pneumonia in a tertiary care ICU: analysis of risk factors for acquisition and mortality. Acta Clin. Belg. 2005;60(3):114–121. https://doi.org/10.1179/acb.2005.022
X
Referencias
Guzmán-Herrador B, Molina CD, Allam MF, Navajas RFC. Independent risk factors associated with hospital-acquired pneumonia in an adult ICU: 4-year prospective cohort study in a university reference hospital. J. Public Health (Oxf) 2016;38(2):378–383. https://doi.org/10.1093/pubmed/fdv042
X
Referencias
Blot S. Antiseptic mouthwash, the nitrate-nitrite-nitric oxide pathway, and hospital mortality: a hypothesis generating review. Intensive Care Med. 2021;47(1):28–38. https://doi.org/10.1007/s00134-020-06276-z
X
Referencias
Otero ML, Menezes RC, Ferreira IBB, Issa FL, Agareno G, Carmo TA, et al. Factors associated with mortality in critically ill patients diagnosed with hospital acquired infections. Infect Drug Resist. 2020;13:2811–7. https://doi.org//10.2147/IDR.S347794
X
Referencias
World Health Organization. Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level. World Health Organization. [Internet] 2016 [cited 2022 May 27]. Available from: https://www.who.int/publications/i/item/9789241549929
X
Referencias
Allegranzi B, Nejad SB, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. The Lancet. 2011;377(9761). https://doi.org//10.1016/S0140-6736(10)61458-4
X
Referencias
Magill SS, Hellinger W, Cohen J, Kay R, Bailey C, Boland B, et al. Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol. 2012;33(3):283-91. https://doi.org//10.1086/664048
X
Referencias
Goodman KE, Simner PJ, Tamma PD, Milstone AM. Infection control implications of heterogeneous resistance mechanisms in carbapenem- resistant Enterobacteriaceae (CRE). Expert Rev Anti Infect Ther. 2016;14(1):95-108. https://doi.org/10.1586/14787210.2016.1106940
X
Referencias
Bork JT, Leekha S, Claeys K, Seung H, Tripoli M, Amoroso A, et al. Change in hospital antibiotic use and acquisition of multidrug-resistant gram-negative organisms after the onset of coronavirus disease 2019. Infect Control Hosp Epidemiol. 2021;42(9):1115-7. https://doi.org/10.1017/ice.2020.1360
X
Referencias
Garcia-Vidal C, Sanjuan G, Moreno-Garcia E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021; 27(1):83–88. https://doi.org//10.1016/j.cmi.2020.07.041
X
Referencias
Kumar G, Adams A, Hererra M, Rojas ER, Singh V, Sakhuja A, et al. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int J Infect Dis. 2021; 104:287–92. https://doi.org//10.1016/j.ijid.2020.11.135
X
Referencias
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-32. https://doi.org//10.1016/j.ajic.2008.03.002
X
Referencias
Wintaco LM, Quintero-Lesmes DC, Vargas JA, Barrera DM, Palacio LN, Granados U, et al. Base Datos IAAS - Analysis Healthcare Infections before and during of COVID-19 pandemic in a Colombian hospital. GitLab 2023. https://gitlab.com/investigaciones1/IAAS
X
Referencias
Aranaz-Andrés JM, Aibar-Remón C, Limón-Ramírez R, Amarillac A, Restrepo FR, Urroze O, et al. Diseño del estudio IBEAS: prevalencia de efectos adversos en hospitales de Latinoamérica. Revista de calidad asistencial. 2011;26(3):194-200. https://doi.org//10.1016/j.cali.2010.12.001
X
Referencias
Luna CM, Rodriguez-Noriega E, Bavestrello L, Guzmán-Blanco M. Gram-negative infections in adult intensive care units of latin america and the caribbean. Crit Care Res and Pract. 2014;480463. https://doi.org/10.1155/2014/480463
X
Referencias
Subbalakshmi E. Surveillance of Hospital Acquired Infection from Frequently Handled Surfaces in a Tertiary Care Teaching Hospital. Int. J.Curr. Microbiol. 2018;7(2) 860-866. https://doi.org/10.20546/ijcmas.2018.702.108
X
Referencias
Cortes JA, Leal AL, Montañez AM, Buitrago G, Castillo JS, Guzman L. Frequency of microorganisms isolated in patients with bacteremia in intensive care units in Colombia and their resistance profiles. Braz J Infect Dis. 2013;17(3):346–52. https://doi.org/10.1016/j.bjid.2012.10.022
X
Referencias
Leal AL, Cortés JA, Arias G, Ovalle MV, Saavedra SY, Buitrago G, et al. Emergence of resistance to third generation cephalosporins by Enterobacteriaceae causing community-onset urinary tract infections in hospitals in Colombia]. Enferm Infecc Microbiol Clin. 2013;31(5):298-303. https://doi.org/10.1016/j.eimc.2012.04.007
X
Referencias
Stewart S, Robertson C, Kennedy S, Kavanagh K, Haahr L, Manoukian S, et al. Personalized infection prevention and control: identifying patients at risk of healthcare-associated infection. J Hosp Infect. 2021;114:32-42. https://doi.org/10.1016/j.jhin.2021.03.032
X
Referencias
Leal AL, Álvarez CA, Cortes J, Camacho G, Sáenz V. Boletín informativo GREBO. [Internet] 2017 [cited 2018 Oct 14]; Available from: www.grupogrebo.org
X
Referencias
Sligl WI, Dragan T, Smith SW. Nosocomial Gram-negative bacteremia in intensive care: Epidemiology, antimicrobial susceptibilities, and outcomes. Int J of Infect Dis. 2015; 37:129-34. https://doi.org/10.1016/j.ijid.2015.06.024
X
Referencias
Dananché C, Bénet T, Allaouchiche B, Hernu R, Argaud L, Dauwalder O, et al. Targeted screening for third-generation cephalosporin-resistant Enterobacteriaceae carriage among patients admitted to intensive care units: a quasi-experimental study. Crit Care. 2015;19(1):38. https://doi.org/10.1186/s13054-015-0754-7
X
Referencias
Langford BJ, Brown KA, Diong C, Marchand-Austin A, Adomako K, Saedi A, et al. The Benefits and Harms of Antibiotic Prophylaxis for Urinary Tract Infection in Older Adults. Clin Infect Dis. 2021;73(3):e782-e791. https://doi.org/10.1093/cid/ciab116
X
Referencias
Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ. 2010;340:c2096. https://doi.org/10.1136/bmj.c2096
X
Referencias
Gamalathge PU, Kularatna S, Carter HE, Senanayake S, Graves N. Cost-effectiveness of interventions to reduce the risk of healthcare-acquired infections in middle-income countries: A systematic review. J Infect Prev. 2019;20(6):266-273. https://doi.org/10.1177/1757177419852662
X
Referencias
Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763
X
Referencias
Voidazan S, Albu S, Toth R, Grigorescu B, Rachita A, Moldovan I. Healthcare Associated Infections-A New Pathology in Medical Practice? Int J Environ Res Public Health. 2020;17(3):760. https://doi.org/10.3390/ijerph17030760
X
Referencias
Hurford A, Morris AM, Fisman DN, Wu J. Linking antimicrobial prescribing to antimicrobial resistance in the ICU: Before and after an antimicrobial stewardship program. Epidemics. 2012;4(4):203–10. https://doi.org/10.1016/j.epidem.2012.12.001
X
Referencias
Katsios CM, Burry L, Nelson S, Jivraj T, Lapinsky SE, Wax RS, et al. An antimicrobial stewardship program improves antimicrobial treatment by culture site and the quality of antimicrobial prescribing in critically ill patients. Crit Care. 2012;16(6):1-9. https://doi.org/10.1186/cc11854
-
Prevention of Hospital Acquired Infections: A practical Guide, G Ducel, J Gabry, L Nicolle. 2nd edition, 2002, Published by World Health Organization WHO/CDS/CSR/EPH/, Geneva; 2002.
-
Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N Engl J Med. 2014;370(13):1198–208. https://doi.org/10.1056/NEJMoa1306801
-
Centers for Disease Control and Prevention. National and State Healthcare-associated Infections Progress Report. [Internet] [cited 2016 May 27]. Available from: https://www.cdc.gov/hai/data/portal/progress-report.html
-
Myny D, Depuydt P, Colardyn F, Blot S. Ventilator-associated pneumonia in a tertiary care ICU: analysis of risk factors for acquisition and mortality. Acta Clin. Belg. 2005;60(3):114–121. https://doi.org/10.1179/acb.2005.022
-
Guzmán-Herrador B, Molina CD, Allam MF, Navajas RFC. Independent risk factors associated with hospital-acquired pneumonia in an adult ICU: 4-year prospective cohort study in a university reference hospital. J. Public Health (Oxf) 2016;38(2):378–383. https://doi.org/10.1093/pubmed/fdv042
-
Blot S. Antiseptic mouthwash, the nitrate-nitrite-nitric oxide pathway, and hospital mortality: a hypothesis generating review. Intensive Care Med. 2021;47(1):28–38. https://doi.org/10.1007/s00134-020-06276-z
-
Otero ML, Menezes RC, Ferreira IBB, Issa FL, Agareno G, Carmo TA, et al. Factors associated with mortality in critically ill patients diagnosed with hospital acquired infections. Infect Drug Resist. 2020;13:2811–7. https://doi.org//10.2147/IDR.S347794
-
World Health Organization. Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level. World Health Organization. [Internet] 2016 [cited 2022 May 27]. Available from: https://www.who.int/publications/i/item/9789241549929
-
Allegranzi B, Nejad SB, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. The Lancet. 2011;377(9761). https://doi.org//10.1016/S0140-6736(10)61458-4
-
Magill SS, Hellinger W, Cohen J, Kay R, Bailey C, Boland B, et al. Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol. 2012;33(3):283-91. https://doi.org//10.1086/664048
-
Instituto Nacional de Salud. Dirección de redes en salud pública. Informe de resultados de la vigilancia por laboratorio de resistencia antimicrobiana en infecciones asociadas a la atención en salud (IAAS 2018) Consulta: agosto 22, 2020. https://www.ins.gov.co/buscador-eventos/Informacin%20de%20laboratorio/Informe-vigilancia-por-laboratorio-resistencia-antimicrobiana-y-whonet-IAAS-2018.pdf
-
Instituto Nacional de Salud. Dirección de redes en salud pública. Informe de resultados de la vigilancia por laboratorio de resistencia antimicrobiana en infecciones asociadas a la atención en salud (IAAS 2018) Consulta: agosto 22, 2020. https://www.ins.gov.co/buscador-eventos/Informacin%20de%20laboratorio/Informe-vigilancia-por-laboratorio-resistencia-antimicrobiana-y-whonet-IAAS-2018.pdf
-
Goodman KE, Simner PJ, Tamma PD, Milstone AM. Infection control implications of heterogeneous resistance mechanisms in carbapenem- resistant Enterobacteriaceae (CRE). Expert Rev Anti Infect Ther. 2016;14(1):95-108. https://doi.org/10.1586/14787210.2016.1106940
-
Canton R, Gijon D, Ruiz-Garbajosa P. Antimicrobial resistance in ICUs: An update in the light of the COVID-19 pandemic. Curr Opin Crit Care. 2020;26(5):433-41. https://doi.org/10.1097/MCC.0000000000000755
-
Bork JT, Leekha S, Claeys K, Seung H, Tripoli M, Amoroso A, et al. Change in hospital antibiotic use and acquisition of multidrug-resistant gram-negative organisms after the onset of coronavirus disease 2019. Infect Control Hosp Epidemiol. 2021;42(9):1115-7. https://doi.org/10.1017/ice.2020.1360
-
Garcia-Vidal C, Sanjuan G, Moreno-Garcia E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021; 27(1):83–88. https://doi.org//10.1016/j.cmi.2020.07.041
-
Kumar G, Adams A, Hererra M, Rojas ER, Singh V, Sakhuja A, et al. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int J Infect Dis. 2021; 104:287–92. https://doi.org//10.1016/j.ijid.2020.11.135
-
O'Toole RF. The interface between COVID-19 and bacterial healthcare-associated infections. Clin Microbiol Infect. 2021;27(12):1772-1776. https://doi.org/10.1016/j.cmi.2021.06.001
-
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-32. https://doi.org//10.1016/j.ajic.2008.03.002
-
Lan L, Xu D, Ye G, et al. Positive RT-PCR test results in patients recovered from COVID19. JAMA. 2020;323(15)1502-1503. https://doi.org//10.1001/jama.2020.2783
-
Stelling JM, O'Brien TF. Surveillance of antimicrobial resistance: the WHONET program. Clinical infectious diseases. 1997;24(1):S157-S68. https://doi.org//10.1093/clinids/24.supplement_1.s157
-
Wintaco LM, Quintero-Lesmes DC, Vargas JA, Barrera DM, Palacio LN, Granados U, et al. Base Datos IAAS - Analysis Healthcare Infections before and during of COVID-19 pandemic in a Colombian hospital. GitLab 2023. https://gitlab.com/investigaciones1/IAAS
-
Aranaz-Andrés JM, Aibar-Remón C, Limón-Ramírez R, Amarillac A, Restrepo FR, Urroze O, et al. Diseño del estudio IBEAS: prevalencia de efectos adversos en hospitales de Latinoamérica. Revista de calidad asistencial. 2011;26(3):194-200. https://doi.org//10.1016/j.cali.2010.12.001
-
Luna CM, Rodriguez-Noriega E, Bavestrello L, Guzmán-Blanco M. Gram-negative infections in adult intensive care units of latin america and the caribbean. Crit Care Res and Pract. 2014;480463. https://doi.org/10.1155/2014/480463
-
Sikora A, Zahra F. Nosocomial Infections. In: StatPearls. Treasure Island (FL): StatPearls Publishing. [Internet] 2024 [Updated 2023 Apr 27] Available from: https://www.ncbi.nlm.nih.gov/books/NBK559312/.
-
Subbalakshmi E. Surveillance of Hospital Acquired Infection from Frequently Handled Surfaces in a Tertiary Care Teaching Hospital. Int. J.Curr. Microbiol. 2018;7(2) 860-866. https://doi.org/10.20546/ijcmas.2018.702.108
-
Cortes JA, Leal AL, Montañez AM, Buitrago G, Castillo JS, Guzman L. Frequency of microorganisms isolated in patients with bacteremia in intensive care units in Colombia and their resistance profiles. Braz J Infect Dis. 2013;17(3):346–52. https://doi.org/10.1016/j.bjid.2012.10.022
-
Leal AL, Cortés JA, Arias G, Ovalle MV, Saavedra SY, Buitrago G, et al. Emergence of resistance to third generation cephalosporins by Enterobacteriaceae causing community-onset urinary tract infections in hospitals in Colombia]. Enferm Infecc Microbiol Clin. 2013;31(5):298-303. https://doi.org/10.1016/j.eimc.2012.04.007
-
Stewart S, Robertson C, Kennedy S, Kavanagh K, Haahr L, Manoukian S, et al. Personalized infection prevention and control: identifying patients at risk of healthcare-associated infection. J Hosp Infect. 2021;114:32-42. https://doi.org/10.1016/j.jhin.2021.03.032
-
Leal AL, Álvarez CA, Cortes J, Camacho G, Sáenz V. Boletín informativo GREBO. [Internet] 2017 [cited 2018 Oct 14]; Available from: www.grupogrebo.org
-
Sligl WI, Dragan T, Smith SW. Nosocomial Gram-negative bacteremia in intensive care: Epidemiology, antimicrobial susceptibilities, and outcomes. Int J of Infect Dis. 2015; 37:129-34. https://doi.org/10.1016/j.ijid.2015.06.024
-
Dananché C, Bénet T, Allaouchiche B, Hernu R, Argaud L, Dauwalder O, et al. Targeted screening for third-generation cephalosporin-resistant Enterobacteriaceae carriage among patients admitted to intensive care units: a quasi-experimental study. Crit Care. 2015;19(1):38. https://doi.org/10.1186/s13054-015-0754-7
-
Langford BJ, Brown KA, Diong C, Marchand-Austin A, Adomako K, Saedi A, et al. The Benefits and Harms of Antibiotic Prophylaxis for Urinary Tract Infection in Older Adults. Clin Infect Dis. 2021;73(3):e782-e791. https://doi.org/10.1093/cid/ciab116
-
Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ. 2010;340:c2096. https://doi.org/10.1136/bmj.c2096
-
Waller TA, Pantin SAL, Yenior AL, Pujalte GGA. Urinary Tract Infection Antibiotic Resistance in the United States. Prim Care. 2018;45(3):455–66. https://doi.org/10.1016/j.pop.2018.05.005
-
Gamalathge PU, Kularatna S, Carter HE, Senanayake S, Graves N. Cost-effectiveness of interventions to reduce the risk of healthcare-acquired infections in middle-income countries: A systematic review. J Infect Prev. 2019;20(6):266-273. https://doi.org/10.1177/1757177419852662
-
Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. https://doi.org/10.1001/jamainternmed.2013.9763
-
Voidazan S, Albu S, Toth R, Grigorescu B, Rachita A, Moldovan I. Healthcare Associated Infections-A New Pathology in Medical Practice? Int J Environ Res Public Health. 2020;17(3):760. https://doi.org/10.3390/ijerph17030760
-
Hurford A, Morris AM, Fisman DN, Wu J. Linking antimicrobial prescribing to antimicrobial resistance in the ICU: Before and after an antimicrobial stewardship program. Epidemics. 2012;4(4):203–10. https://doi.org/10.1016/j.epidem.2012.12.001
-
Katsios CM, Burry L, Nelson S, Jivraj T, Lapinsky SE, Wax RS, et al. An antimicrobial stewardship program improves antimicrobial treatment by culture site and the quality of antimicrobial prescribing in critically ill patients. Crit Care. 2012;16(6):1-9. https://doi.org/10.1186/cc11854